



现代地质 ›› 2025, Vol. 39 ›› Issue (02): 248-262.DOI: 10.19657/j.geoscience.1000-8527.2024.133
出版日期:2025-04-10
发布日期:2025-05-08
通信作者:
尹 烁,男,副研究员,1991年出生,主要从事砂岩铀矿研究工作。Email: yinshuo@ecut.edu.cn。作者简介:金伟国,男,硕士研究生,1998年出生,主要从事砂岩型铀矿研究工作。Email: 963920206@qq.com。
基金资助:
JIN Weiguo1,2( ), YIN Shuo1(
), YIN Shuo1( ), WANG Qingfei1,3, PAN Jiayong1
), WANG Qingfei1,3, PAN Jiayong1
Published:2025-04-10
Online:2025-05-08
摘要:
针对砂岩型铀矿中微生物与矿物互作机制证据链薄弱的关键科学问题,本研究以松辽盆地海力锦矿床姚家组下段含铀独居石为研究对象,通过微区矿物学分析揭示其溶蚀过程与铀活化机理。基于钻孔岩心系统取样,采用FIB-TEM联用技术首次在独居石溶蚀界面识别出氢铀云母与沥青铀矿纳米矿物组合,结合电子探针原位分析发现溶蚀相独居石平均损失75%初始铀。研究揭示:(1)石英包裹效应导致独居石选择性溶蚀特征,开放体系下溶蚀强度提升3~4个数量级;(2)溶蚀界面纳米矿物相的定向分布指示微生物代谢产生的有机酸主导磷铀耦合释放过程;(3)建立的生物膜催化动力学模型显示,微生物介导的界面反应使铀活化至沉淀。该研究不仅为微生物与纳米矿物间协同成矿机制提供了关键的显微尺度证据,而且对于促进铀矿地质学研究向更精细的微观领域深入拓展具有重要的意义。
中图分类号:
金伟国, 尹烁, 王庆飞, 潘家永. FIB-TEM解析微生物铀矿化:以松辽盆地海力锦砂岩型铀矿为例[J]. 现代地质, 2025, 39(02): 248-262.
JIN Weiguo, YIN Shuo, WANG Qingfei, PAN Jiayong. Deciphering Microbial-Mediated Uranium Mineralization via FIB-TEM Nanotomography: A Case Study from the Hailijin Deposit, Songliao Basin[J]. Geoscience, 2025, 39(02): 248-262.

图1 松辽盆地南部构造单元及矿床地质简图(据文献[7]修改) (a)松辽盆地一级构造单元区划图;(b)松辽盆地南部构造单元区划图;(c)海力锦矿床及其邻近地区地质简图;Ⅰ.开鲁坳陷区; Ⅱ.西南隆起区; Ⅲ.西部斜坡区; Ⅳ.中央坳陷区; Ⅴ.西缘斜坡带; Ⅰ1.陆家堡凹陷; Ⅰ2.哲中凹陷; Ⅰ3.乌兰花凸起; Ⅰ4.钱家店凹陷; Ⅱ1.白音花凹陷; Ⅱ2.三棵树鼻状凸起; Ⅱ3.瞻榆凹陷; Ⅱ4.架玛吐凸起; Ⅱ5.巴彦塔拉凸起; Ⅱ6.大林凹陷; Ⅱ7.金宝屯凹陷; Ⅱ8.宝格吐凹陷; Ⅱ9.张强凹陷; Ⅱ10.安乐凹陷
Fig.1 Simplified geological map for the Hailijin deposit and tectonic units of the southern Songliao Basin (modified from reference [7])

图2 海力锦铀矿床姚家组A-A'质剖面简图(据文献[11,13]修改)
Fig.2 A-A' simplified geological profile of Yaojia Formation in Hailijin uranium deposit (modified from references [11,13])
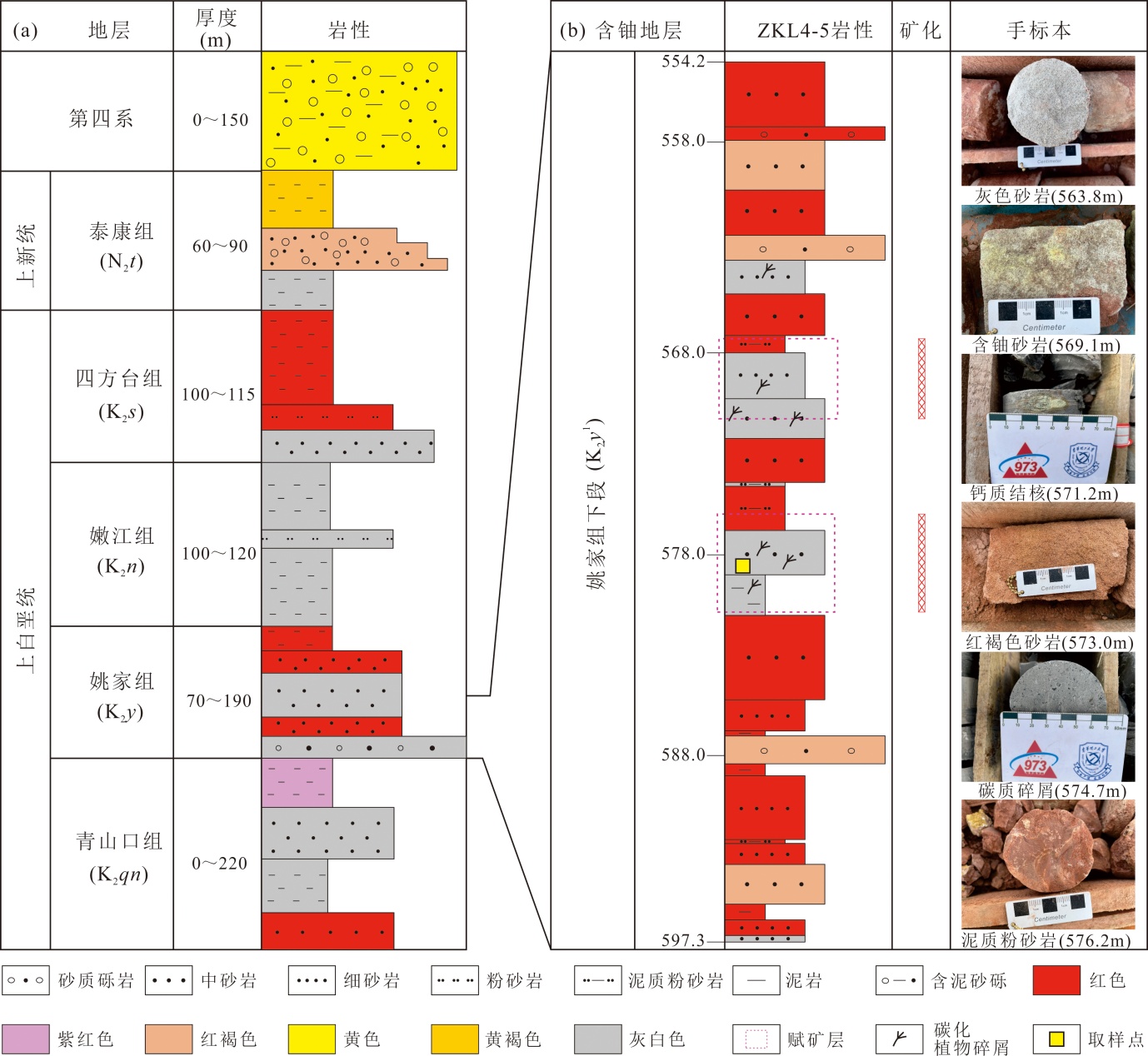
图3 海力锦铀矿床地层相关厚度及ZKL4-5钻孔柱状图(据文献[12-13]修改) (a)海力锦矿床地层及相关厚度;(b)岩心ZKL4-5钻探获得的含铀地层岩性、深度、铀矿分布及手标本照片
Fig.3 Regional strata, related thickness and ZKL4-5 sedimentary column of drill core in the Hailijin uranium deposit (modified from references [12-13])
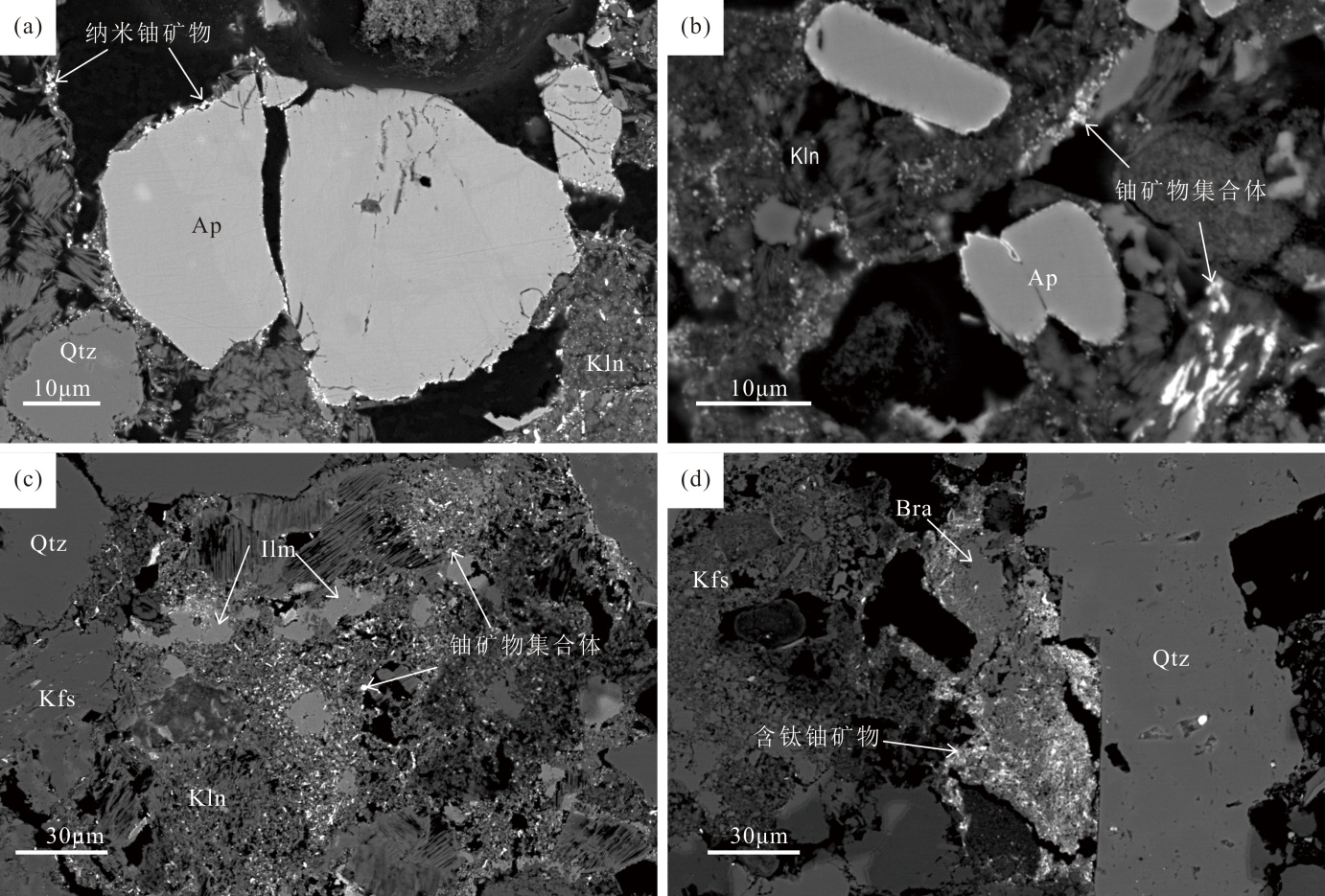
图4 铀矿物赋存相态特征 (a)磷灰石和相邻高岭石边缘吸附纳米铀矿物;(b)纳米铀矿物集合体出现在多孔的高岭石表面;(c)碎屑状铀矿物集合体分布在石英、钾长石和钛铁矿颗粒孔隙间;(d)含钛铀矿物填充蚀变铁钛氧化物孔隙;Ap.磷灰石;Ilm.钛铁矿;Kfs.钾长石;Kln.高岭石;Qtz.石英
Fig.4 Paragenetic types and occurrence modes of uranium minerals

图5 独居石与磷灰石背散射电子显微对比 (a)—(c)石英包裹的碎屑独居石颗粒;(d)—(f)被严重溶蚀的独居石,表面见大量暗色点状、针状孔隙;(g)—(i)磷灰石在高对比度下结构较为完整;Ap.磷灰石;Ilm.钛铁矿;Kfs.钾长石;Kln.高岭石;Mnz.独居石;Qtz.石英
Fig.5 Comparative backscattered electron (BSE) microscopy of monazite-apatite assemblages
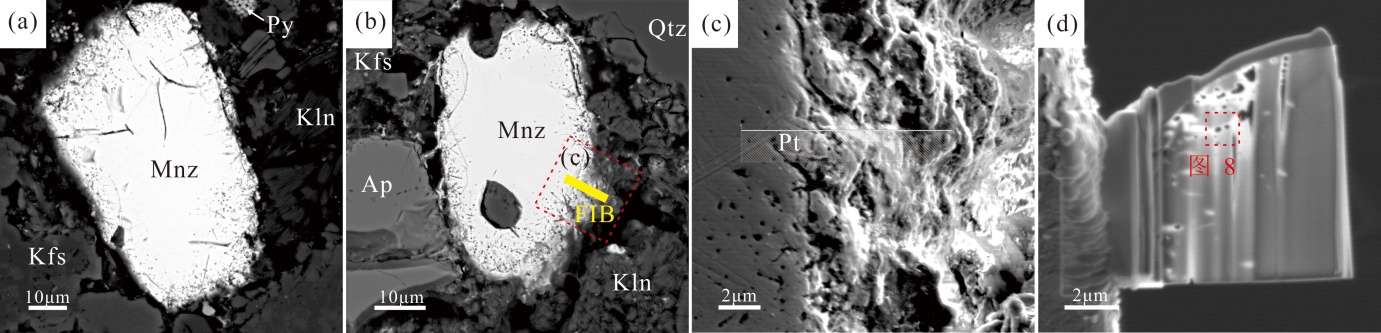
图7 溶蚀独居石的BSE显微结构与FIB制样定位 (a)(b)溶蚀独居石背散射图片,其中(b)黄色区域为FIB切片位置;(c)FIB切片前独居石表面纳米孔隙形貌特征;(d)独居石FIB薄片(~100 nm);Ap.磷灰石;Kfs.钾长石;Kln.高岭石;Mnz.独居石;Py.黄铁矿;Qtz.石英
Fig.7 BSE microtextures of corroded monazite with FIB-milled cross-section markers

图9 独居石纳米孔隙的明场透射电子显微解析 (a) 溶蚀独居石纳米孔隙透射电镜明场图像;(b)为(a)纳米孔隙选区分析位置透射电镜亮场图像;(c) 独居石和相邻氢铀云母的晶格条纹图像,其中右上角为所选区域独居石选区衍射图;(d)为(c)所选区域的氢铀云母选区衍射图;(e) 氢铀云母晶格条纹图像,其中右上角为所选区域氢铀云母多晶环状衍射图;(f) 沥青铀矿与独居石和氢铀云母相邻的晶格条纹图像,其中右上角为所选区域沥青铀矿多晶环状衍射图;(g)为图(f)中所选区域的沥青铀矿选区衍射图;(h)为图(f)中所选区域的氢铀云母选区衍射图;(i) 沥青铀矿和相邻独居石的晶格条纹图像,其中右上角为所选区域沥青铀矿选区衍射图,晶体衍射图像中每组数字为该矿物对应晶面的晶面指数;Ckv.氢铀云母;Mnz.独居石;Pit.沥青铀矿
Fig.9 Bright-field TEM characterization of nanoscale porosity in monazite
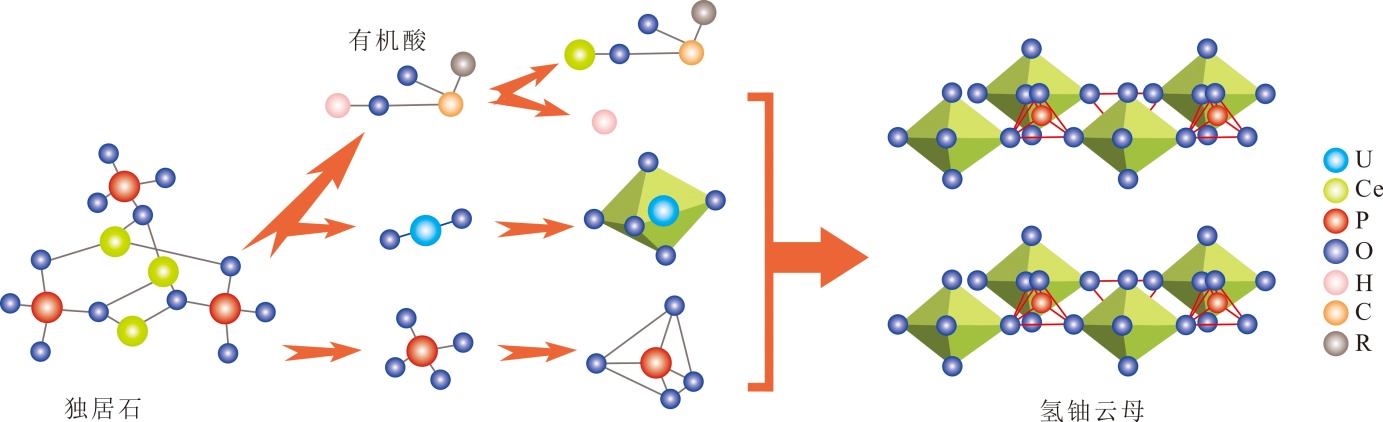
图10 有机酸介导的独居石溶解与氢铀云母结晶耦合机制(据文献[40,47-49]修改) 有机酸结构通式R-COOH,R表示不同性质的酸酚
Fig.10 Coupled mechanism of monazite dissolution and meta-autunite crystallization mediated by organic acids (modified from references [40,47-49])

图11 独居石与氢铀云母晶体原子模型 (a)CePO4沿[111]晶带轴方向原子排列模型;(b)沿[111]晶带轴方向独居石透射电镜高分辨晶格条纹图像;(c)图(b)中独居石选区分析的Ce原子排列;(d)H3O(U2O)PO4(H2O)3沿[111]晶带轴方向原子排列模型;(e)沿[111]晶带轴方向氢铀云母透射电镜高分辨晶格条纹图像;(f)图(e)中氢铀云母选区分析的U原子排列;Ckv.氢铀云母;Mnz.独居石
Fig.11 Atomic-scale structural models at monazite and chernikovite interface

图12 砂岩型铀矿中独居石生物地球化学溶蚀动力学模型(据文献[14]修改) (a)海力锦铀矿床成矿示意图;(b)同沉积过程中碎屑独居石与其他碎屑矿物组合;(c)有机酸溶蚀碎屑独居石;(d)早期成岩阶段碎屑独居石、长石、钛铁矿遭受蚀变;(e)独居石进一步溶蚀结晶形成氢铀云母纳米矿物;(f)晚期成岩阶段独居石呈溶蚀残留状态存在;(g)弱碱性流体溶解氢云母释放铀并还原结晶形成沥青铀矿纳米矿物;Ap.磷灰石;Ckv.氢铀云母;Ilm.钛铁矿;Kfs.钾长石;Kln.高岭石;Mnz.独居石;Pit.沥青铀矿;Qtz.石英;Tdo.多孔氧化钛
Fig.12 Bio-geochemical dissolution kinetics model of monazite in sandstone-hosted uranium deposits (modified from reference [14])

图13 溶蚀过程中独居石主微量元素演化趋势 (a)碎屑独居石到溶蚀独居石的UO2含量变化特征;(b)碎屑独居石到溶蚀独居石的Th/U含量变化特征
Fig.13 Evolutionary trends of major trace elements in monazite during dissolution
| 主量元素 (%) | 样品编号 | 最大值 | 最小值 | 平均值 | ||||
|---|---|---|---|---|---|---|---|---|
| 1-1 | 1-2 | 1-3 | 1-4 | 1-5 | ||||
| SiO2 | 0.27 | 0.26 | 0.31 | - | - | 0.31 | 0.26 | 0.27 |
| ThO2 | 3.87 | 3.18 | 3.43 | 4.18 | 3.72 | 4.18 | 3.`18 | 3.72 |
| UO2 | 0.32 | 0.30 | 0.36 | 0.26 | 0.25 | 0.36 | 0.25 | 0.30 |
| P2O5 | 29.00 | 29.05 | 29.38 | 28.84 | 29.00 | 29.38 | 28.84 | 29.00 |
| La2O3 | 11.45 | 11.20 | 10.73 | 10.34 | 10.70 | 11.45 | 10.34 | 10.73 |
| Ce2O3 | 33.56 | 32.53 | 34.30 | 38.64 | 37.82 | 38.64 | 32.53 | 34.30 |
| Pr2O3 | 4.06 | 3.20 | 3.87 | 3.36 | 3.75 | 4.06 | 3.20 | 3.75 |
| Nd2O3 | 11.87 | 11.50 | 12.95 | 11.53 | 11.22 | 12.95 | 11.22 | 11.53 |
| Sm2O3 | 2.36 | 2.06 | 2.60 | 1.58 | 1.73 | 2.60 | 1.58 | 2.06 |
| Eu2O3 | 0.62 | 0.81 | 0.54 | 0.69 | 0.76 | 0.81 | 0.54 | 0.69 |
| Gd2O3 | 1.29 | 2.30 | 1.36 | 0.73 | 0.70 | 2.30 | 0.70 | 1.29 |
| Dy2O3 | 0.78 | 0.82 | 0.09 | 0.05 | 0.23 | 0.82 | 0.05 | 0.23 |
| Y2O3 | 1.11 | 1.32 | 0.21 | 0.16 | 0.12 | 1.32 | 0.12 | 0.21 |
| Lu2O3 | - | 0.05 | 0.14 | 0.19 | 0.20 | 0.20 | 0.05 | 0.17 |
| SO3 | 0.03 | 0.03 | - | 0.01 | 0.02 | 0.03 | 0.01 | 0.03 |
| CaO | 0.80 | 0.81 | 0.80 | 0.04 | - | 0.81 | 0.04 | 0.80 |
| Total | 101.39 | 99.44 | 101.50 | 100.59 | 100.20 | 101.50 | 99.44 | 100.59 |
| Th/U | 12.01 | 10.50 | 9.60 | 16.14 | 15.11 | 16.14 | 9.60 | 12.01 |
| 主量元素 (%) | 样品编号 | 最大值 | 最小值 | 平均值 | ||||
| 2-1 | 2-2 | 2-3 | 2-4 | 2-5 | ||||
| SiO2 | 0.28 | 0.26 | 0.24 | 0.29 | 0.19 | 0.29 | 0.19 | 0.26 |
| ThO2 | 4.00 | 4.08 | 4.03 | 4.21 | 4.09 | 4.21 | 4.00 | 4.08 |
| UO2 | 0.06 | 0.05 | 0.10 | 0.05 | 0.10 | 0.10 | 0.05 | 0.06 |
| P2O5 | 28.85 | 28.84 | 28.75 | 28.51 | 28.67 | 28.85 | 28.51 | 28.75 |
| La2O3 | 11.15 | 10.82 | 11.09 | 11.22 | 12.82 | 12.82 | 10.82 | 11.15 |
| Ce2O3 | 31.15 | 32.10 | 31.35 | 32.24 | 33.79 | 33.79 | 31.15 | 32.10 |
| Pr2O3 | 3.97 | 4.05 | 3.87 | 3.60 | 4.03 | 4.05 | 3.60 | 3.97 |
| Nd2O3 | 12.51 | 12.52 | 11.61 | 12.26 | 11.29 | 12.52 | 11.29 | 12.26 |
| Sm2O3 | 2.32 | 2.11 | 2.43 | 2.25 | 1.88 | 2.43 | 1.88 | 2.25 |
| Eu2O3 | 0.66 | 0.44 | 0.80 | 0.61 | 0.62 | 0.80 | 0.44 | 0.62 |
| Gd2O3 | 1.75 | 1.62 | 2.14 | 1.50 | 1.95 | 2.14 | 1.50 | 1.75 |
| Dy2O3 | 0.35 | 0.42 | - | 0.67 | 0.18 | 0.67 | 0.18 | 0.39 |
| Y2O3 | 1.67 | 1.85 | 2.06 | 1.69 | 1.31 | 2.06 | 1.31 | 1.69 |
| Lu2O3 | 0.20 | 0.10 | 0.10 | - | - | 0.20 | 0.10 | 0.10 |
| SO3 | - | - | - | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| CaO | 0.80 | 0.77 | 0.91 | 0.79 | 0.51 | 0.91 | 0.51 | 0.79 |
| Total | 99.73 | 100.03 | 99.47 | 99.90 | 100.45 | 100.45 | 99.47 | 99.90 |
| Th/U | 64.52 | 75.46 | 41.52 | 84.26 | 42.65 | 84.26 | 41.52 | 64.52 |
表1 独居石微区电子探针分析数据集
Table 1 Major and trace element concentrations of monazite by electron probe microanalysis (EPMA)
| 主量元素 (%) | 样品编号 | 最大值 | 最小值 | 平均值 | ||||
|---|---|---|---|---|---|---|---|---|
| 1-1 | 1-2 | 1-3 | 1-4 | 1-5 | ||||
| SiO2 | 0.27 | 0.26 | 0.31 | - | - | 0.31 | 0.26 | 0.27 |
| ThO2 | 3.87 | 3.18 | 3.43 | 4.18 | 3.72 | 4.18 | 3.`18 | 3.72 |
| UO2 | 0.32 | 0.30 | 0.36 | 0.26 | 0.25 | 0.36 | 0.25 | 0.30 |
| P2O5 | 29.00 | 29.05 | 29.38 | 28.84 | 29.00 | 29.38 | 28.84 | 29.00 |
| La2O3 | 11.45 | 11.20 | 10.73 | 10.34 | 10.70 | 11.45 | 10.34 | 10.73 |
| Ce2O3 | 33.56 | 32.53 | 34.30 | 38.64 | 37.82 | 38.64 | 32.53 | 34.30 |
| Pr2O3 | 4.06 | 3.20 | 3.87 | 3.36 | 3.75 | 4.06 | 3.20 | 3.75 |
| Nd2O3 | 11.87 | 11.50 | 12.95 | 11.53 | 11.22 | 12.95 | 11.22 | 11.53 |
| Sm2O3 | 2.36 | 2.06 | 2.60 | 1.58 | 1.73 | 2.60 | 1.58 | 2.06 |
| Eu2O3 | 0.62 | 0.81 | 0.54 | 0.69 | 0.76 | 0.81 | 0.54 | 0.69 |
| Gd2O3 | 1.29 | 2.30 | 1.36 | 0.73 | 0.70 | 2.30 | 0.70 | 1.29 |
| Dy2O3 | 0.78 | 0.82 | 0.09 | 0.05 | 0.23 | 0.82 | 0.05 | 0.23 |
| Y2O3 | 1.11 | 1.32 | 0.21 | 0.16 | 0.12 | 1.32 | 0.12 | 0.21 |
| Lu2O3 | - | 0.05 | 0.14 | 0.19 | 0.20 | 0.20 | 0.05 | 0.17 |
| SO3 | 0.03 | 0.03 | - | 0.01 | 0.02 | 0.03 | 0.01 | 0.03 |
| CaO | 0.80 | 0.81 | 0.80 | 0.04 | - | 0.81 | 0.04 | 0.80 |
| Total | 101.39 | 99.44 | 101.50 | 100.59 | 100.20 | 101.50 | 99.44 | 100.59 |
| Th/U | 12.01 | 10.50 | 9.60 | 16.14 | 15.11 | 16.14 | 9.60 | 12.01 |
| 主量元素 (%) | 样品编号 | 最大值 | 最小值 | 平均值 | ||||
| 2-1 | 2-2 | 2-3 | 2-4 | 2-5 | ||||
| SiO2 | 0.28 | 0.26 | 0.24 | 0.29 | 0.19 | 0.29 | 0.19 | 0.26 |
| ThO2 | 4.00 | 4.08 | 4.03 | 4.21 | 4.09 | 4.21 | 4.00 | 4.08 |
| UO2 | 0.06 | 0.05 | 0.10 | 0.05 | 0.10 | 0.10 | 0.05 | 0.06 |
| P2O5 | 28.85 | 28.84 | 28.75 | 28.51 | 28.67 | 28.85 | 28.51 | 28.75 |
| La2O3 | 11.15 | 10.82 | 11.09 | 11.22 | 12.82 | 12.82 | 10.82 | 11.15 |
| Ce2O3 | 31.15 | 32.10 | 31.35 | 32.24 | 33.79 | 33.79 | 31.15 | 32.10 |
| Pr2O3 | 3.97 | 4.05 | 3.87 | 3.60 | 4.03 | 4.05 | 3.60 | 3.97 |
| Nd2O3 | 12.51 | 12.52 | 11.61 | 12.26 | 11.29 | 12.52 | 11.29 | 12.26 |
| Sm2O3 | 2.32 | 2.11 | 2.43 | 2.25 | 1.88 | 2.43 | 1.88 | 2.25 |
| Eu2O3 | 0.66 | 0.44 | 0.80 | 0.61 | 0.62 | 0.80 | 0.44 | 0.62 |
| Gd2O3 | 1.75 | 1.62 | 2.14 | 1.50 | 1.95 | 2.14 | 1.50 | 1.75 |
| Dy2O3 | 0.35 | 0.42 | - | 0.67 | 0.18 | 0.67 | 0.18 | 0.39 |
| Y2O3 | 1.67 | 1.85 | 2.06 | 1.69 | 1.31 | 2.06 | 1.31 | 1.69 |
| Lu2O3 | 0.20 | 0.10 | 0.10 | - | - | 0.20 | 0.10 | 0.10 |
| SO3 | - | - | - | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| CaO | 0.80 | 0.77 | 0.91 | 0.79 | 0.51 | 0.91 | 0.51 | 0.79 |
| Total | 99.73 | 100.03 | 99.47 | 99.90 | 100.45 | 100.45 | 99.47 | 99.90 |
| Th/U | 64.52 | 75.46 | 41.52 | 84.26 | 42.65 | 84.26 | 41.52 | 64.52 |
| [1] | TIEH T T, LEDGER E B, ROWE M W. Release of uranium from granitic rocks during in situ weathering and initial erosion (central Texas)[J]. Chemical Geology, 1980, 29(1/2/3/4): 227-248. |
| [2] | ZIELINSKI R A. Uranium mobility during interaction of rhyolitic obsidian, perlite and felsite with alkaline carbonate solution: T=120 ℃, P=210 kg/cm2[J]. Chemical Geology, 1979, 27(1/2): 47-63. |
| [3] | ZIELINSKI R A. Tuffaceous sediments as source rocks for uranium: A case study of the White River Formation, Wyoming[J]. Journal of Geochemical Exploration, 1983, 18(3): 285-306. |
| [4] | ROSHOLT J N, NOBLE D C. Loss of uranium from crystallized silicic volcanic rocks[J]. Earth and Planetary Science Letters, 1969, 6(4): 268-270. |
| [5] | MOYEN J F, CUNEY M, BARATOUX D, et al. Multi-scale spatial distribution of K, Th and U in an Archaean potassic granite: A case study from the Heerenveen batholith, Barberton Granite-Greenstone Terrain, South Africa[J]. South African Journal of Geology, 2021, 124(1): 53-86. |
| [6] | GUTHRIE V A, KLEEMAN J D. Changing uranium distributions during weathering of granite[J]. Chemical Geology, 1986, 54(1/2): 113-126. |
| [7] | 臧亚辉, 李继木, 宁君, 等. 松辽盆地南部海力锦地区上白垩统姚家组砂岩物源示踪及其构造背景综合研究:来自岩石地球化学及锆石U-Pb年代学的制约[J]. 地质科技通报, 2023, 42(5): 175-190. |
| [8] | 田明明, 李子颖, 张云龙, 等. 松辽盆地海力锦铀矿床黄铁矿微量元素、硫同位素特征及其对成矿流体性质的指示[J]. 铀矿地质, 2024, 40(1): 119-128. |
| [9] | 宁君, 李继木, 夏菲, 等. 松辽盆地南部海力锦铀矿床地质特征及成因探讨[J]. 东华理工大学学报(自然科学版), 2023, 46(3): 223-232. |
| [10] | 宁君, 李继木, 卢天军, 等. 松辽盆地南部海力锦铀矿床姚家组下段黏土矿物特征及其铀成矿意义[J]. 铀矿地质, 2023, 39(2): 188-201. |
| [11] | 臧亚辉, 姜山, 李继木, 等. 松辽盆地南部海力锦铀矿床铀矿物赋存状态及富集机理:试论“潮汐式”成矿作用过程[J]. 大地构造与成矿学, 2023, 47(3): 546-569. |
| [12] | TIAN M M, LI Z Y, ZHANG Y L, et al. Genetic mechanism of tabular-shaped orebody of the hailijin sandstone-type uranium deposit in the Songliao basin: Constraints on the clay mineralogy of ore-bearing sandstone[J]. Minerals, 2023, 13(10): 1324. |
| [13] | YAN Z B, ZHANG W W, XIA F, et al. Uranium pre-concentration in sandstone-hosted U deposits: A case study from the hailijin ore field, SW Songliao basin, NE China[J]. Ore Geology Reviews, 2023, 161: 105661. |
| [14] | YIN S, YAN Z B, FU J L, et al. Polymorphic transformations of titanium oxides contribute to economic uranium mineralization in sandstone[J]. Geology, 2024, 52(7): 481-485. |
| [15] | 张红雨, 杨立明, 苏犁, 等. LA-ICP-MS独居石的U(Th)-Pb年龄精确测定方法及地质意义探究[J]. 现代地质, 2023, 37(2): 443-462. |
| [16] | 李厚民, 李立兴, 李以科, 等. 内蒙古白云鄂博铁-铌-稀土矿床矿化蚀变矿物组合及流体组成[J]. 现代地质, 2024, 38(1): 13-24. |
| [17] | 宓奎峰, 杨艳, 颜廷杰, 等. 内蒙古沙麦钨矿区高分异花岗岩独居石U-Pb定年及成矿意义[J]. 现代地质, 2020, 34(3): 504-513. |
| [18] | HECHT L, CUNEY M. Hydrothermal alteration of monazite in the Precambrian crystalline basement of the Athabasca Basin (Saskatchewan, Canada): Implications for the formation of unconformity-related uranium deposits[J]. Mineralium Deposita, 2000, 35(8): 791-795. |
| [19] | WAGANI I, PAGEL M, JANOTS E, et al. Detrital monazite in the tim mersoi basin, Niger: Provenance and contribution to the uranium budget in siliciclastic sediments[J]. The Canadian Mineralogist, 2011, 49(2): 487-501. |
| [20] | AMBOH TIFANG J. Preliminary survey for uranium in Cretaceous sandstones of bonako-sole areas, ngondo complex, southwestern Cameroon[J]. Earth Sciences, 2017, 6(1): 1. |
| [21] | 王海涛. 开鲁坳陷姚家组下段沉积特征与铀成矿作用[J]. 东华理工大学学报(自然科学版), 2022, 45(3): 223-233. |
| [22] | 吴根耀. 白垩纪:中国及邻区板块构造演化的一个重要变换期[J]. 中国地质, 2006, 33(1): 64-77. |
| [23] | REN J Y, TAMAKI K, LI S T, et al. Late Mesozoic and Cenozoic rifting and its dynamic setting in Eastern China and adjacent areas[J]. Tectonophysics, 2002, 344(3/4): 175-205. |
| [24] | LI Z Q, CHEN J L, ZOU H, et al. Mesozoic-Cenozoic tectonic evolution and dynamics of the Songliao Basin, NE Asia: Implications for the closure of the Paleo-Asian Ocean and Mongol-Okhotsk Ocean and subduction of the Paleo-Pacific Ocean[J]. Earth-Science Reviews, 2021, 218: 103471-108252. |
| [25] | WANG P J, MATTERN F, DIDENKO N A, et al. Tectonics and cycle system of the Cretaceous Songliao basin: An inverted active continental margin basin[J]. Earth-Science Reviews, 2016, 159(1): 82-102. |
| [26] | SONG Y, STEPASHKO A, LIU K Y, et al. Post-rift tectonic history of the Songliao basin, NE China: Cooling events and post-rift unconformities driven by orogenic pulses from plate boundaries[J]. Journal of Geophysical Research, 2018, 123(3): 2363-2395. |
| [27] | 宁君, 夏菲, 聂逢君, 等. 浅析松辽盆地南部姚下段灰色砂体与铀成矿关系[J]. 东华理工大学学报(自然科学版), 2018, 41(4): 336-342. |
| [28] | KETTANAH Y A, ISMAIL S A. Heavy mineral concentrations in the sandstones of Amij Formation with particular emphasis on the mineral chemistry and petrographic characteristics of monazite, western desert of Iraq[J]. Journal of African Earth Sciences, 2016, 123: 350-369. |
| [29] | LINTHOUT K. Tripartite division of the system 2REPPO4-CaTh(PO4)2-2ThSiO4, discreditation of brabantite, and recognition of cheralite as the Name for members dominated by CaTh(PO4)2[J]. The Canadian Mineralogist, 2007, 45(3): 503-508. |
| [30] | MORTON A C, HALLSWORTH C. Chapter 7 stability of detrital heavy minerals during burial diagenesis[M]// Heavy Minerals in Use. Amsterdam: Elsevier, 2007: 215-245. |
| [31] | ALLAZ J, SELLECK B, WILLIAMS M L, et al. Microprobe analysis and dating of monazite from the Potsdam formation, New York: A progressive record of chemical reaction and fluid interaction[J]. American Mineralogist, 2013, 98(7): 1106-1119. |
| [32] | RICHARD A, MONTEL J M, LEBORGNE R, et al. Monazite alteration in H2O±HCl±NaCl±CaCl2 fluids at 150 ℃ and psat: Implications for uranium deposits[J]. Minerals, 2015, 5(4): 693-706. |
| [33] | ZHAO L, CAI C F, JIN R S, et al. Mineralogical and geochemical evidence for biogenic and petroleum-related uranium mineralization in the Qianjiadian deposit, NE China[J]. Ore Geology Reviews, 2018, 101: 273-292. |
| [34] | BHATTACHARYYA A, CAMPBELL K M, KELLY S D, et al. Biogenic non-crystalline U(IV) revealed as major component in uranium ore deposits[J]. Nature Communications, 2017, 8(1): 15538. |
| [35] | WORTMANN U G, BERNASCONI S M, BÖTTCHER M E. Hypersulfidic deep biosphere indicates extreme sulfur isotope fractionation during single-step microbial sulfate reduction[J]. Geology, 2001, 29(7): 647. |
| [36] |
SHEN J B, YUAN L X, ZHANG J L, et al. Phosphorus dynamics: From soil to plant[J]. Plant Physiology, 2011, 156(3): 997-1005.
DOI PMID |
| [37] |
CORBETT M K, EKSTEEN J J, NIU X Z, et al. Interactions of phosphate solubilising microorganisms with natural rare-earth phosphate minerals: A study utilizing Western Australian monazite[J]. Bioprocess and Biosystems Engineering, 2017, 40(6): 929-942.
DOI PMID |
| [38] |
BRISSON V L, ZHUANG W Q, ALVAREZ COHEN L.Bioleaching of rare earth elements from monazite sand[J]. Biotechnology and Bioengineering, 2016, 113(2): 339-348.
DOI PMID |
| [39] | HE Y L, MA L Y, LIANG X L, et al. Resistant rare earth phosphates as possible sources of environmental dissolved rare earth elements: Insights from experimental bio-weathering of xenotime and monazite[J]. Chemical Geology, 2024, 661(9): 122186. |
| [40] | SON H J, PARK G T, CHA M S, et al. Solubilization of insoluble inorganic phosphates by a novel salt- and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere[J]. Bioresource Technology, 2006, 97(2): 204-210. |
| [41] | NI Y X, HUGHES J M, MARIANO A N. Crystal chemistry of the monazite and xenotime structures[J]. American Mineralogist, 1995, 80(1/2): 21-26. |
| [42] | FOUGEROUSE D, REDDY S M, SEYDOUX-GUILLAUME A M, et al. Mechanical twinning of monazite expels radiogenic lead[J]. Geology, 2021, 49(4): 417-421. |
| [43] | SHEN Y H, ZHENG X Y, WANG X Y, et al. The biomineralization process of uranium(VI) by Saccharomyces cerevisiae-transformation from amorphous U(VI) to crystalline chernikovite[J]. Applied Microbiology and Biotechnology, 2018, 102(9): 4217-4229. |
| [44] | ZHENG X Y, SHEN Y H, WANG X Y, et al. Effect of pH on uranium(VI) biosorption and biomineralization by Saccharomyces cerevisiae[J]. Chemosphere, 2018, 203: 109-116. |
| [45] | WANG P P, DONG F Q, WANG X H, et al. Effects of riboflavin and AQS as electron shuttles on U(vi) reduction and precipitation by Shewanella putrefaciens[J]. RSC Advances, 2018, 8(54): 30692-30700. |
| [46] | HU N, LI K, SUI Y, et al. Utilization of phosphate rock as a sole source of phosphorus for uranium biomineralization mediated by Penicillium funiculosum[J]. RSC Advances, 2018, 8(24): 13459-13465. |
| [47] | YU Q H, YUAN Y H, FENG L J, et al. Highly efficient immobilization of environmental uranium contamination with Pseudomonas stutzeri by biosorption, biomineralization, and bioreduction[J]. Journal of Hazardous Materials, 2022, 424: 127758. |
| [48] | ZHENG X Y, WANG X Y, SHEN Y H, et al. Biosorption and biomineralization of uranium(VI) by saccharomyces cerevisiae:Crystal formation of chernikovite[J]. Chemosphere, 2017, 175: 161-169. |
| [49] | NIE X, DONG F, BIAN L, et al. Uranium Binding on Landoltia punctata as a Result of Formation of Insoluble Nano-U (VI) and U (IV) Phosphate Minerals[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(2): 1494-1502. |
| [50] | 沈扬皓. 啤酒酵母和拟棘壳孢属菌与铀的相互作用机理研究[D]. 兰州: 兰州大学, 2021. |
| [51] | TANG X, LI Q L, ZHANG B, et al. The chemical state and occupancy of radiogenic Pb, and crystallinity of RW-1 monazite revealed by XPS and TEM[J]. Minerals, 2020, 10(6): 504. |
| [52] | SHIN D, KIM J, KIM B S, et al. Use of phosphate solubilizing bacteria to leach rare earth elements from monazite-bearing ore[J]. Minerals, 2015, 5(2): 189-202. |
| [53] | XIE J C, LIN J F, ZHOU X H. pH-dependent microbial reduction of uranium(VI) in carbonate-free solutions: UV-vis, XPS, TEM, and thermodynamic studies[J]. Environmental Science and Pollution Research, 2018, 25(22): 22308-22317. |
| [54] | FANIZZA M F, YOON H, ZHANG C Y, et al. Pore-scale evaluation of uranyl phosphate precipitation in a model groundwater system[J]. Water Resources Research, 2013, 49(2): 874-890. |
| [55] | SINGH A, ULRICH K U, GIAMMAR D E. Impact of phosphate on U(VI) immobilization in the presence of goethite[J]. Geochimica et Cosmochimica Acta, 2010, 74(22): 6324-6343. |
| [1] | 张万良, 李余亮. 华南信江盆地砂岩型铀成矿控矿因素[J]. 现代地质, 2024, 38(04): 1109-1120. |
| [2] | 王战永, 隆兆笃, 解波, 孙悦, 李巨初, 向杰, 范永宏. 四川冕西岩体岩石地球化学特征及铀成矿条件分析[J]. 现代地质, 2022, 36(06): 1465-1474. |
| [3] | 冯博, 段培新, 程旭, 卢辉雄, 李瑞炜, 张恩, 汪冰. 高分五号航天高光谱遥感技术在甘肃龙首山铀矿找矿中的应用[J]. 现代地质, 2022, 36(06): 1594-1604. |
| [4] | 吕永华, 苗爱生, 王果, 李曙光, 高峥嵘. 二连盆地那仁地区铀成矿地质条件分析及找矿预测[J]. 现代地质, 2022, 36(02): 662-671. |
| [5] | 胡妍, 胡永兴, 张翔, 杨涛, 欧扬剑. 鄂尔多斯盆地西南缘镇原地区砂岩型铀矿元素地球化学特征及地质意义[J]. 现代地质, 2020, 34(06): 1153-1165. |
| [6] | 刘子杰, 邹明亮, 孙远强, 李杰, 陈琪, 朱鹏飞, 张涛. 桂北苗儿山岩体中段向阳坪铀矿床F7构造带三维地质模型的构建与应用[J]. 现代地质, 2020, 34(04): 653-662. |
| [7] | 黄志新, 李子颖, 蔡煜琦, 朱斌, 任全. 华北陆块北缘哈毕力格铀矿床S、Pb同位素组成及对成矿作用的指示[J]. 现代地质, 2020, 34(03): 588-597. |
| [8] | 王伟, 王生云, 刘涛, 李天石, 陈云杰, 马骊, 赵如意, 宋振涛. 甘肃红石泉伟晶岩型铀矿床地质、地球化学特征及成因探讨[J]. 现代地质, 2020, 34(02): 244-253. |
| [9] | 孙岳, 潘家永, 陈正乐, 韩凤彬, 刘文恒, 肖伟峰. 乌兹别克中卡兹库姆地貌特征对砂岩型铀矿赋存的制约[J]. 现代地质, 2019, 33(05): 1070-1078. |
| [10] | 徐浩, 张闯, 庞雅庆, 曹豪杰, 刘佳林, 刘文泉. 广东长排铀矿床成矿流体特征[J]. 现代地质, 2018, 32(05): 902-912. |
| [11] | 刘刚, 刘家军, 袁峰, 张帅, 沙亚洲, 张宏远, 王功文. 陕西小花岔铀矿床岩浆演化及其对铀成矿作用的制约[J]. 现代地质, 2017, 31(05): 990-1005. |
| [12] | 张万良, 李子颖, 阙足双, 林锦荣. 水力压裂技术对江西相山热液铀矿成因的启示[J]. 现代地质, 2017, 31(03): 521-533. |
| [13] | 李西得,易超,高贺伟,陈心路,张康,王明太. 鄂尔多斯盆地东北部直罗组古层间氧化带 形成机制探讨[J]. 现代地质, 2016, 30(4): 739-747. |
| [14] | 李子颖,张万良. 江西相山矿田主要铀矿化类型及其地球化学特征对比研究[J]. 现代地质, 2016, 30(1): 1-16. |
| [15] | 姚宏鑫,吕古贤,聂江涛,郑光高,曹小兵,. 江西相山铀矿田邹家山铀矿床蚀变特征及热液来源[J]. 现代地质, 2013, 27(2): 332-338. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||