



现代地质 ›› 2025, Vol. 39 ›› Issue (03): 637-647.DOI: 10.19657/j.geoscience.1000-8527.2024.132
出版日期:2025-06-10
发布日期:2025-07-03
作者简介:闫丽霞,女,博士,讲师,1991年出生,主要从事环境矿物学和矿物表界面性质研究工作。Email:yanlixia0507@163.com。
基金资助:
YAN Lixia( ), REN Zihe, ZHANG Bingchang, LIU Ting, PAN Xumei
), REN Zihe, ZHANG Bingchang, LIU Ting, PAN Xumei
Published:2025-06-10
Online:2025-07-03
摘要:
水铁矿相转化影响近地表铁氧化物矿物组成及元素归趋,具有重要的地球化学意义,被广泛关注。随着研究深入,研究方法不断革新。本文结合水铁矿相转化的研究进展,综合分析在该研究中的常用研究方法,包括光谱学(X射线衍射、红外光谱、拉曼光谱、穆斯堡尔谱、X射线吸收精细结构谱等)、形貌学(扫描电镜及透射电镜)、元素分析及磁性分析等手段,探究这些常用方法在水铁矿相转化过程中的应用(定性及定量),提出不同研究方法的优势及其局限性。最后,对今后水铁矿研究中原位测试方法的应用进行展望。本研究为探究近地表环境铁氧化物矿物的转变过程提供基础信息。
中图分类号:
闫丽霞, 任子贺, 张丙昌, 刘婷, 潘须眉. 水铁矿相转化研究常用方法综述[J]. 现代地质, 2025, 39(03): 637-647.
YAN Lixia, REN Zihe, ZHANG Bingchang, LIU Ting, PAN Xumei. Common Study Methods for Ferrihydrite Transformation: A Review[J]. Geoscience, 2025, 39(03): 637-647.

图2 混合样品的红外光谱(Fh=Fhy, Gt=Gth, L=Lep)[22] (a)Fhy和Gt;(b)Fhy和nGt;(c)Fhy和L;(d)Gt和L;(e)Fhy和Mag;(f)Gt和Mag;(g)L和Mag
Fig. 2 FTIR spectra of the mixed samples[22]
| 铁氧化物 | 测试温度(K) | IS(mm/s) | QS(mm/s) | BHF(T) |
|---|---|---|---|---|
| 2线水铁矿 | 295.0 | 0.24 | 0.79 | - |
| 4.2 | 0.24 | -0.01 | 47.0 | |
| 6线水铁矿 | 292.0 | 0.24 | 0.72 | - |
| 4.2 | 0.25 | -0.06 | 50.0 | |
| 赤铁矿 | 295.0 | 0.37 | -0.20 | 50.75 |
| 4.2 | 0.49 | 0.41 | 54.17 | |
| 针铁矿 | 295.0 | 0.37 | -0.26 | 38.0 |
| 4.2 | 0.48 | -0.25 | 50.60 | |
| 磁铁矿 | 295.0 | 0.26 | 0 | 49.0 |
| 0.6 | ≤|0.02| | 46.0 | ||
| 纤铁矿 | 294.0 | 0.37 | 0.53 | - |
| 4.2 | 0.47 | 0.02 | 45.8 | |
| 施式矿物 | 295.0 | 0.39 | 0.64 | - |
| 4.2 | 0.49 | -0.37 | 45.6 |
表1 常见铁氧化物矿物的穆斯堡尔参数[10]
Table 1 Mössbauer parameters of the common iron oxides[10]
| 铁氧化物 | 测试温度(K) | IS(mm/s) | QS(mm/s) | BHF(T) |
|---|---|---|---|---|
| 2线水铁矿 | 295.0 | 0.24 | 0.79 | - |
| 4.2 | 0.24 | -0.01 | 47.0 | |
| 6线水铁矿 | 292.0 | 0.24 | 0.72 | - |
| 4.2 | 0.25 | -0.06 | 50.0 | |
| 赤铁矿 | 295.0 | 0.37 | -0.20 | 50.75 |
| 4.2 | 0.49 | 0.41 | 54.17 | |
| 针铁矿 | 295.0 | 0.37 | -0.26 | 38.0 |
| 4.2 | 0.48 | -0.25 | 50.60 | |
| 磁铁矿 | 295.0 | 0.26 | 0 | 49.0 |
| 0.6 | ≤|0.02| | 46.0 | ||
| 纤铁矿 | 294.0 | 0.37 | 0.53 | - |
| 4.2 | 0.47 | 0.02 | 45.8 | |
| 施式矿物 | 295.0 | 0.39 | 0.64 | - |
| 4.2 | 0.49 | -0.37 | 45.6 |

图6 Fhy转化前后As的XANES图谱(a)、EXAFS图谱(b)、傅里叶变换图谱(c)[12]
Fig.6 XANES (a), EXAFS (b), and Fourier transform spectra (c) of As before and after the phase transformation of Fhy[12]
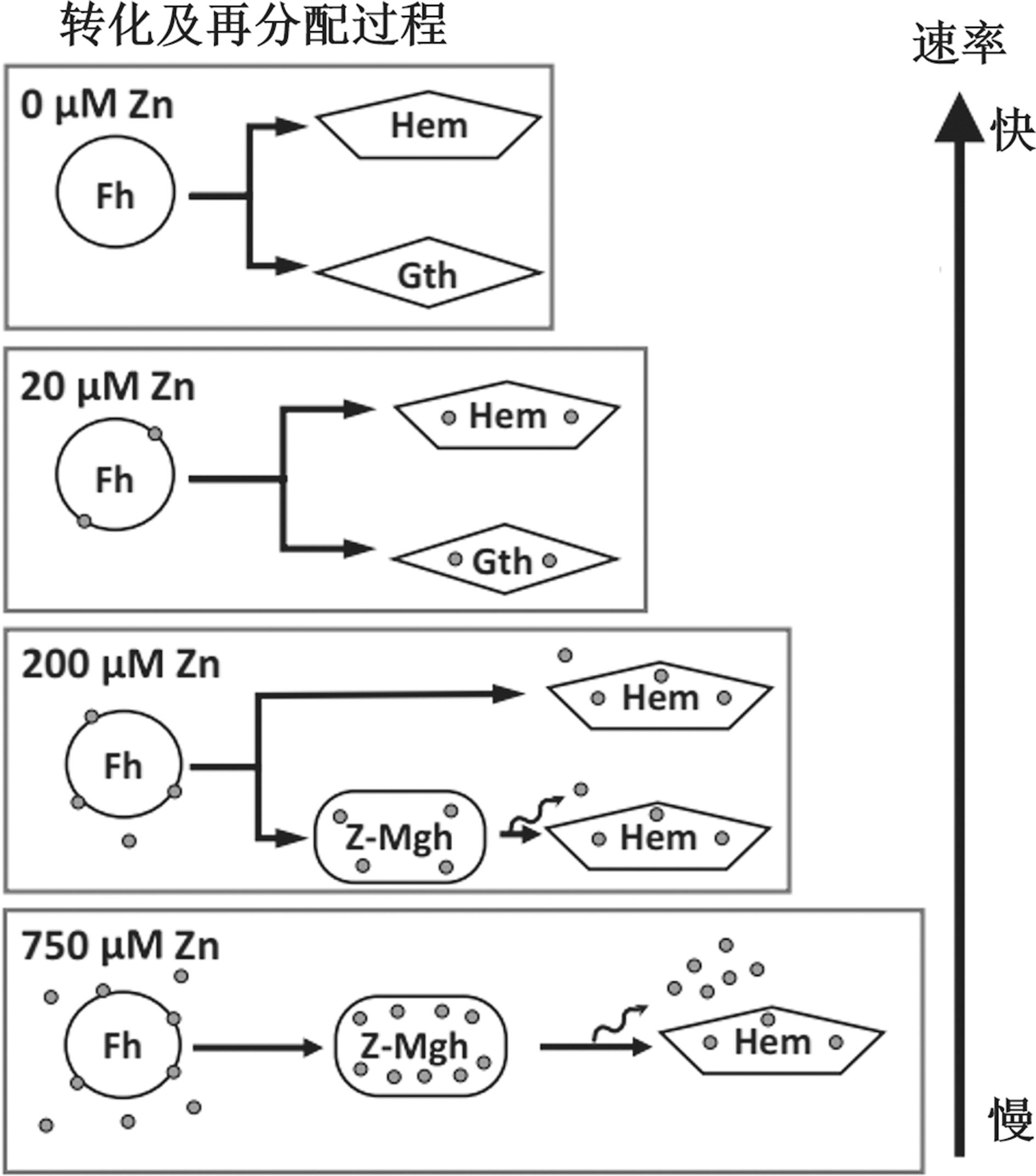
图9 不同Zn含量条件下Fhy的转化过程、产物及Zn的分布状态[56]
Fig.9 Phase transformation process and the transformed product of Fhy in the presence of Zn, and the distribution of Zn in the transformation process[56]
| [1] | LIANG Y, JIN J Z, WEI Z Y, et al. Complexation mechanism of Pb2+ at the ferrihydrite-water interface: The role of Al-substitution[J]. Chemosphere, 2022, 307: 135627. |
| [2] | EYNDE E V, HIEMSTRA T, COMANS R. Interaction of Zn with ferrihydrite and its cooperative binding in the presence of PO4-[J]. Geochimica et Cosmochimica Acta, 2022, 320. |
| [3] | DING Z, SUN G Z, FU F J, et al. Phase transformation of Cr(Ⅵ)-adsorbed ferrihydrite in the presence of Mn(Ⅱ): Fate of Mn(Ⅱ)and Cr(Ⅵ)[J]. Journal of Environmental Sciences, 2022(3): 9. |
| [4] | LI Y, YANG M J, PENTRAL M P, et al. Carbonate-Enhanced Transformation of Ferrihydrite to Hematite[J]. Environmental Science & Technology, 2020, 54(21): 13701-13708. |
| [5] | YAN L X, ZHU R L, LIU J, et al. Effects of Fullerol and Graphene Oxide on the Phase Transformation of Two-Line Ferrihydrite[J]. ACS Earth and Space Chemistry, 2020, 4: 335-344. |
| [6] | LIU J, SHENG A X, LI X X, et al. Understanding the Importance of Labile Fe(III) during Fe(II)-Catalyzed Transformation of Metastable Iron Oxyhydroxides[J]. Environmental Science & Technology, 2022, 56(6): 3801-3811. |
| [7] | SHU ZP, LIU L H, TAN W F, et al. Solar Irradiation Induced Transformation of Ferrihydrite in the Presence of Aqueous Fe2+[J]. Environmental Science & Technology, 2019, 53. |
| [8] | DAS S, HENDRY M, Essilfie-Dughan J. Transformation of Two-line Fenihydrite to Goethite and Hematite as a Function of pH and Temperature[J]. Environmental Science & Technology, 2011, 45(1): 268-275. |
| [9] | DAS S, HENDRY M J, ESSILFIE-DUGHAN J. Effects of Adsorbed Arsenate on the Rate of Transformation of 2-Line Ferrihydrite at pH 10[J]. Environmental Science & Technology, 2011, 45(13): 5557-5563. |
| [10] | CORNELL R M, SCHWERTMANN U. The iron oxides: Structure, properties, reactions, occurrence and uses 2nd (Ed)[M]. Wiley-VCH, 2003. |
| [11] | JOHNSTON J H, LEWIS D G. A detailed study of the transformation of ferrihydrite to hematite in an aqueous medium at 92℃[J]. Geochim. Cosmochim. Acta, 1983, 47: 1823-1831. |
| [12] | DAS S, ESSILFIE-DUGHAN J, HENDRY M J. Arsenate partitioning from ferrihydrite to hematite: Spectroscopic evidence[J]. American Mineralogist, 2014, 99(4): 749-754. |
| [13] | YANG L, STEEFEL C I, MARCUS M A, et al. Kinetics of Fe(II)-catalyzed Transformation of Ferrihydrite Under Anaerobic Dynamic Flow Conditions[J]. Environmental Science & Technology, 2017, 44(14): 5469-5475. |
| [14] | DAS S, HENDRY M J. Characterization of hematite nanoparticles synthesized via two different pathways[J]. Journal of Nanoparticle Research, 2014, 16(8): 2535. |
| [15] | NAMDURI H, NASRAZADANI S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry[J]. Corrosion Science, 2008, 50(9): 2493-2497. |
| [16] | NASRAZADANI S. The application of infrared spectroscopy to a study of phosphoric and tannic acids interactions with magnetite (Fe3O4), goethite (α-FeOOH) and lepidocrocite (γ-FeOOH)[J]. Corrosion Science, 1997, 39(10): 1845-1859. |
| [17] | NASRAZADANI S, RAMAN A. The application of infrared spectroscopy to the study of rust systems-II. Study of cation deficiency in magnetite (Fe3O4) produced during its transformation to maghemite (γ-Fe2O3) and hematite (α-Fe2O3)[J]. Corrosion Science, 1993, 34(8): 1355-1365. |
| [18] | CARABANTE I, GRAHN M, HOLMGREN A, et al. Influence of Zn(II) on the Adsorption of Arsenate onto Ferrihydrite[J]. Environmental Science & Technology, 2012, 46(24): 13152-13159. |
| [19] | RUDOLPH M, ERLER J, PEUKER U A. A TGA-FTIR perspective of fatty acid adsorbed on magnetite nanoparticles-Decomposition steps and magnetite reduction[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012, 397: 16-23. |
| [20] | YANG K, PENG H B, WEN Y H, et al. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles[J]. Applied Surface Science, 2010, 256(10): 3093-3097. |
| [21] |
PEAK D, FORD R G, SPARKS D L. An in Situ ATR-FTIR Investigation of Sulfate Bonding Mechanisms on Goethite[J]. Journal of Colloid and Interface Science, 1999, 218(1): 289-299.
PMID |
| [22] |
XIAO W, JONES A M, COLLINS R N, et al. Use of fourier transform infrared spectroscopy to examine the Fe(II)-Catalyzed transformation of ferrihydrite[J]. Talanta, 2017, 175: 30-37.
DOI PMID |
| [23] |
PARIKH S, CHOROVER J. ATR-FTIR spectroscopy reveals bond formation during bacterial adhesion to iron oxide[J]. Langmuir, 2006, 22: 8492-500.
DOI PMID |
| [24] | RAMAN A, KUBAN B, RAZVAN A. The application of infrared spectroscopy to the study of atmospheric rust systems-I. Standard spectra and illustrative applications to identify rust phases in natural atmospheric corrosion products[J]. Corrosion Science, 1991, 32(12): 1295-1306. |
| [25] | ARAI Y, SPARKS D L. ATR-FTIR Spectroscopic Investigation on Phosphate Adsorption Mechanisms at the Ferrihydrite-Water Interface[J]. Journal of Colloid and Interface Science, 2001, 241(2): 317-326. |
| [26] |
CARABANTE I, GRAHN M, HOLMOREN A, et al. In situ ATR-FTIR studies on the competitive adsorption of arsenate and phosphate on ferrihydrite[J]. Journal of Colloid and Interface Science, 2010, 351(2): 523-531.
DOI PMID |
| [27] | 秦孝荣, 姚玉增, 何宏平, 等. 广东梅州花岗岩风化壳剖面的可见光-短波红外反射光谱特征及其对风化强度的指示[J]. 地球化学, 2020, 49(4): 422-434. |
| [28] | ZHAO L L, HONG H L, LIU J C, et al. Assessing the utility of visible-to-shortwave infrared reflectance spectroscopy for analysis of soil weathering intensity and paleoclimate reconstruction[J]. Palaeogeogr Palaeoclimatol Palaeoecol, 2018, 512: 80-94. |
| [29] | 王小明. 几种亚稳定铁氧化物的结构、形成转化及其表面物理化学特征[D]. 武汉: 华中农业大学, 2015. |
| [30] | MAZZETTI L, THISTLETHWAITE P J. Raman spectra and thermal transformations of ferrihydrite and schwertmannite[J]. Journal of Raman Spectroscopy, 2002, 33(2): 104-111. |
| [31] | LEMINE O M, SAJIEDDINE M, BOUOUDINA M, et al. Rietveld analysis and Mössbauer spectroscopy studies of nanocrystalline hematite α-Fe2O3[J]. Journal of Alloys and Compounds, 2010, 502(2): 279-282. |
| [32] | SCHWERTMANN U, FRIEDL J, STANJEK H, et al. The effect of clay minerals on the formation of goethite and hematite from ferrihydrite after 16 years’ ageing at 25℃ and pH 4-7[J]. Clay Minerals, 2000, 35(4): 613-623. |
| [33] | MARSHALL T A, MORRIS K, LAW G T W, et al. Incorporation of Uranium into Hematite during Crystallization from Ferrihydrite[J]. Environmental science & technology, 2014, 48(7): 3724-3731. |
| [34] |
WANG Z H, XIAO D X, BUSH R T, et al. Coprecipitated arsenate inhibits thermal transformation of 2-line ferrihydrite: Implications for long-term stability of ferrihydrite[J]. Chemosphere, 2015, 122: 88-93.
DOI PMID |
| [35] | CAMPBELL A S, SCHWERTMANN U, STANJEK H, et al. Si Incorporation into Hematite by Heating Si-Ferrihydrite[J]. Langmuir, 2002, 18(21): 7804-7809. |
| [36] | DRITS V A, SAKHAROV B A, SALYN A L, et al. Structural Model for Ferrihydrite[J]. Clay Minerals, 1993, 28: 185-207. |
| [37] | PAKTUNC D, MANCEAU A, DUTRIZAC J. Incorporation of Ge in ferrihydrite: Implications for the structure of ferrihydrite[J]. American Mineralogist, 2013, 98(5/6): 848-858. |
| [38] | LEE S, SHEN Z Z, XU H F. Study on nanophase iron oxyhydroxides in freshwater ferromanganese nodules from Green Bay, Lake Michigan, with implications for the adsorption of As and heavy metals[J]. American Mineralogist, 2016, 101(9): 1986-1995. |
| [39] | SCHWERTMANN U, STANJEK H. Stirring Effects on Properties of Al Goethite Formed from Ferrihydrite[J]. Clays and Clay Minerals, 1998, 46: 317-321. |
| [40] | CORNELL R M, GIOVANOLI R, SCHINDLER P W. Effect of Silicate Species on the Transformation of Ferrihydrite into Goethite and Hematite in Alkaline Media[J]. Clays and Clay Minerals, 1987, 35: 21-28. |
| [41] | SHAW S, PEPPER S E, BRYAN N D, et al. The kinetics and mechanisms of goethite and hematite crystallization under alkaline conditions, and in the presence of phosphate[J]. American Mineralogist, 2005, 90(11/12): 1852-1860. |
| [42] | CORNELL R M. Effect of Simple Sugars on the Alkaline Transformation of Ferrihydrite into Goethite and Hematite[J]. Clays and Clay Minerals, 1985, 33: 219-227. |
| [43] | FISCHER W R, SCHWERTMANN U. The Formation of Hematite from Amorphous Iron(III)Hydroxide[J]. Clays and Clay Minerals, 1975, 23: 33-37. |
| [44] | WOLSKA E, SZAJDA W. The effect of cationic and anionic substitution on the α-(Al, Fe)2O3 lattice parameters[J]. Solid State Ionics, 1988, 28/29/30: 1320-1323. |
| [45] | STANJEK H, WEIDLER P G. The effect of dry heating on the chemistry, surface area, and oxalate solubility of synthetic 2-line and 6-line ferrihydrites[J]. Clay Minerals, 1992, 27(4): 397-411. |
| [46] | DINSEN A, PEDERSEN C T, BENDER K C. The Thermal Conversion of Lepidocrocite (γ-FeOOH) Revisited[J]. Journal of Thermal Analysis and Calorimetry, 2001, 64: 1303-1310. |
| [47] | CORNELL R M, GIOVANOLI R. The influence of copper on the transformation of ferrihydrite (5Fe2O3 · 9H2O) into crystalline products in alkaline media[J]. Polyhedron, 1988, 7(5): 385-391. |
| [48] | CORNELL R M, GIOVANOLI R. Effect of cobalt on the formation of crystalline iron oxides from ferrihydrite in alkaline media[J]. Clays and Clay Minerals, 1989, 37(1): 65-70. |
| [49] | 张美诺, 石康兴, 邱昆峰, 等. 冀东司家营BIF铁矿床磁铁矿类型与成因及其对高品位铁矿化成矿机制的启示[J]. 现代地质, 2025, 39(1): 96-114. |
| [50] | SHEN N M, XIANG X M, SHE X C, et al. Hydrothermal synthesis and characterization of single crystalline Cu-doped α-FeOOH nanowires[J]. Applied Surface Science, 2012, 259: 306-310. |
| [51] | LIU C S, ZHU Z K, LI F B, et al. Fe(II)-induced phase transformation of ferrihydrite: The inhibition effects and stabilization of divalent metal cations[J]. Chemical Geology, 2016, 444: 110-119. |
| [52] | ECHIGO T, MONSEGUE N, ARUGUETE D M, et al. Nanopores in hematite (α-Fe2O3) nanocrystals observed by electron tomography[J]. American Mineralogist, 2013, 98(1): 154-162. |
| [53] | GIOVANOLI R, CORNELL R M. Crystallization of metal substituted ferrihydrites[J]. Journal of Plant Nutrition and Soil Science, 1992, 155: 455-460. |
| [54] | ZHANG T, ZENG X B, ZHANG H. Investigation of synthetic ferrihydrite transformation in soils using two-step sequential extraction and the diffusive gradients in thin films (DGT) technique[J]. Geoderma, 2018, 321: 90-99. |
| [55] | VOELZ J L, JOHNSON N W, CHUN C L, et al. Quantitative dissolution of environmentally accessible iron residing in iron-rich minerals: A review[J]. ACS Earth and Space Chemistry, 2019, 3(8): 1371-1392. |
| [56] | SAKAKIBARA M, TANAKA M, TAKAHASHI Y, et al. Redistribution of Zn during transformation of ferrihydrite: Effects of initial Zn concentration[J]. Chemical Geology, 2019, 522: 121-134. |
| [57] | LIN X M, BURNS R C, LAWRANCE G. Effect of Cadmium(Ii) and anion type on the ageing of ferrihydrite and its subsequent leaching under neutral and alkaline conditions[J]. Water Air and Soil Pollution, 2003, 143: 155-177. |
| [58] | CORNELL R M, GIOVANOLI R, SCHNEIDER W. Effect of cysteine and manganese on the crystallization of noncrystalline iron(III) hydroxide at pH 8[J]. Clays and Clay Minerals, 1990, 38(1): 21-28. |
| [59] | JIANG Z X, LIU Q S, ROBERT A, et al. A new model for transformation of ferrihydrite to hematite in soils and sediments[J]. Geology, 2018, 46(11): 987-990. |
| [60] | LIU Q S, BARRÓN V, TORRENT J, et al. Magnetism of intermediate hydromaghemite in the transformation of 2-line ferrihydrite into hematite and its paleoenvironmental implications[J]. Journal of Geophysical Research, 2008, 113: B01103. |
| [61] | 张文艳, 朱裕振, 刘雪, 等. 山东禹城李屯地区重磁异常特征与找矿预测[J]. 现代地质, 2024, 38(1): 68-76. |
| [62] | 闫丽霞, 任子贺. 碳纳米管对水铁矿相转化的影响[J]. 现代地质, 2025, 38(3): 627-635. |
| [1] | 闫丽霞, 任子贺. 碳纳米管对水铁矿相转化的影响与机制[J]. 现代地质, 2025, 39(03): 628-636. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||