



Geoscience ›› 2021, Vol. 35 ›› Issue (04): 931-939.DOI: 10.19657/j.geoscience.1000-8527.2021.04.04
• Geochemistry • Previous Articles Next Articles
GUAN Junjie1,2( ), LIU Yuyan1,2, LIU Siyuan1,2, CHEN Jiawei1,2(
), LIU Yuyan1,2, LIU Siyuan1,2, CHEN Jiawei1,2( )
)
Received:2019-12-17
Revised:2021-04-19
Online:2021-08-10
Published:2021-09-08
Contact:
CHEN Jiawei
CLC Number:
GUAN Junjie, LIU Yuyan, LIU Siyuan, CHEN Jiawei. Effects of High Temperature and Freeze-thaw Cycle Ageing on Adsorption Performance of Hydrochar and Biochar on Pollutants[J]. Geoscience, 2021, 35(04): 931-939.
| 水热炭 | 灰分*/ % | 元素组分**/% | 原子比** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | H/C | O/C | (O+N) /C | |||
| RH | 16.23 | 51.37 | 6.72 | 41.06 | 0.60 | 1.570 | 0.600 | 0.610 | |
| HRH30 | 15.38 | 49.02 | 6.37 | 44.01 | 0.44 | 1.560 | 0.673 | 0.681 | |
| HRH60 | 16.46 | 49.31 | 6.25 | 43.96 | 0.40 | 1.522 | 0.669 | 0.676 | |
| HRH90 | 15.43 | 49.90 | 6.38 | 43.20 | 0.46 | 1.534 | 0.649 | 0.657 | |
| FRH30 | 15.68 | 50.70 | 6.50 | 42.18 | 0.51 | 1.539 | 0.624 | 0.633 | |
| FRH60 | 16.06 | 50.13 | 6.48 | 42.90 | 0.42 | 1.551 | 0.642 | 0.649 | |
| FRH90 | 15.64 | 49.03 | 6.42 | 44.11 | 0.38 | 1.571 | 0.675 | 0.682 | |
| CS | 7.66 | 52.38 | 6.68 | 39.27 | 1.59 | 1.530 | 0.562 | 0.588 | |
| HCS30 | 7.05 | 52.38 | 6.73 | 39.07 | 1.75 | 1.541 | 0.559 | 0.588 | |
| HCS60 | 6.88 | 51.45 | 6.76 | 40.25 | 1.49 | 1.578 | 0.587 | 0.611 | |
| HCS90 | 6.67 | 51.17 | 6.63 | 40.74 | 1.40 | 1.554 | 0.597 | 0.621 | |
| FCS30 | 5.96 | 51.01 | 6.70 | 40.77 | 1.46 | 1.575 | 0.599 | 0.624 | |
| FCS60 | 7.15 | 51.71 | 6.51 | 40.05 | 1.69 | 1.510 | 0.581 | 0.609 | |
| FCS90 | 6.38 | 51.44 | 6.66 | 40.29 | 1.54 | 1.554 | 0.587 | 0.613 | |
Table 1 Elements analysis of fresh and aged hydrochars
| 水热炭 | 灰分*/ % | 元素组分**/% | 原子比** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | H/C | O/C | (O+N) /C | |||
| RH | 16.23 | 51.37 | 6.72 | 41.06 | 0.60 | 1.570 | 0.600 | 0.610 | |
| HRH30 | 15.38 | 49.02 | 6.37 | 44.01 | 0.44 | 1.560 | 0.673 | 0.681 | |
| HRH60 | 16.46 | 49.31 | 6.25 | 43.96 | 0.40 | 1.522 | 0.669 | 0.676 | |
| HRH90 | 15.43 | 49.90 | 6.38 | 43.20 | 0.46 | 1.534 | 0.649 | 0.657 | |
| FRH30 | 15.68 | 50.70 | 6.50 | 42.18 | 0.51 | 1.539 | 0.624 | 0.633 | |
| FRH60 | 16.06 | 50.13 | 6.48 | 42.90 | 0.42 | 1.551 | 0.642 | 0.649 | |
| FRH90 | 15.64 | 49.03 | 6.42 | 44.11 | 0.38 | 1.571 | 0.675 | 0.682 | |
| CS | 7.66 | 52.38 | 6.68 | 39.27 | 1.59 | 1.530 | 0.562 | 0.588 | |
| HCS30 | 7.05 | 52.38 | 6.73 | 39.07 | 1.75 | 1.541 | 0.559 | 0.588 | |
| HCS60 | 6.88 | 51.45 | 6.76 | 40.25 | 1.49 | 1.578 | 0.587 | 0.611 | |
| HCS90 | 6.67 | 51.17 | 6.63 | 40.74 | 1.40 | 1.554 | 0.597 | 0.621 | |
| FCS30 | 5.96 | 51.01 | 6.70 | 40.77 | 1.46 | 1.575 | 0.599 | 0.624 | |
| FCS60 | 7.15 | 51.71 | 6.51 | 40.05 | 1.69 | 1.510 | 0.581 | 0.609 | |
| FCS90 | 6.38 | 51.44 | 6.66 | 40.29 | 1.54 | 1.554 | 0.587 | 0.613 | |
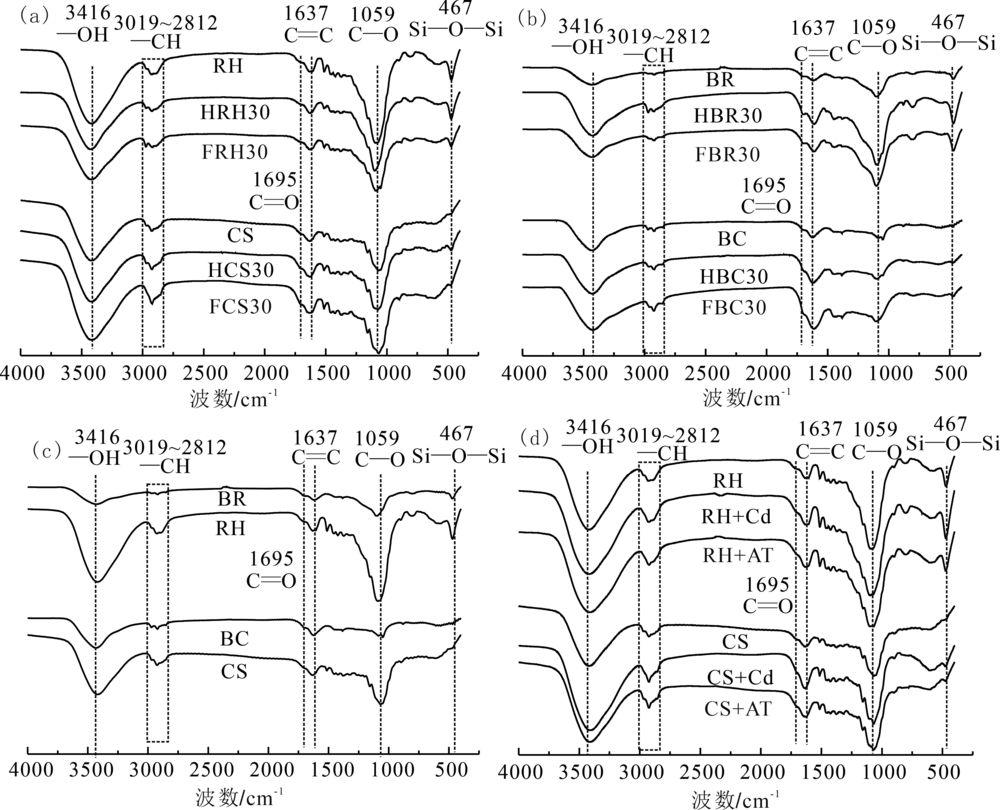
Fig.2 FTIR spectra of the samples (a)fresh and aged hydrochars; (b) fresh and aged biochars;(c) biochars and hydrochars;(d)RH and CS before and after adsorption of atrazine and Cd
| 水热炭 | 准一级动力学方程 | 准二级动力学方程 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k1 | R2 | |||||
| BC | 29.553 | 0.304 | 0.946 | 31.345 | 0.011 | 0.999 | ||||
| BR | 1.126 | 0.412 | 0.678 | 1.597 | 0.176 | 0.997 | ||||
| CS | 2.570 | 0.077 | 0.914 | 2.686 | 0.041 | 0.980 | ||||
| RH | 2.072 | 0.054 | — | 2.128 | 0.069 | 0.992 | ||||
Table 2 Pseudo-first-order and pseudo-second-order kinetic parameters for the sorption of Cd(II) on hydrochars and biochars
| 水热炭 | 准一级动力学方程 | 准二级动力学方程 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k1 | R2 | |||||
| BC | 29.553 | 0.304 | 0.946 | 31.345 | 0.011 | 0.999 | ||||
| BR | 1.126 | 0.412 | 0.678 | 1.597 | 0.176 | 0.997 | ||||
| CS | 2.570 | 0.077 | 0.914 | 2.686 | 0.041 | 0.980 | ||||
| RH | 2.072 | 0.054 | — | 2.128 | 0.069 | 0.992 | ||||
| 水热炭 | 准一级动力学方程 | 准二级动力学方程 | |||||
|---|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k1 | R2 | ||
| BC | 8.453 | 0.0339 | 0.970 | 8.977 | 0.010 | 0.990 | |
| BR | 0.755 | 0.0269 | 0.160 | 0.771 | 0.140 | 0.977 | |
| CS | 1.063 | 0.335 | 0.991 | 1.268 | 0.198 | 0.997 | |
| RH | 1.333 | 1.387 | 0.899 | 1.449 | 0.260 | 0.998 | |
Table 3 Pseudo-first-order and pseudo-second-order kinetic parameters for the sorption of atrazine on hydrochars and biochars
| 水热炭 | 准一级动力学方程 | 准二级动力学方程 | |||||
|---|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k1 | R2 | ||
| BC | 8.453 | 0.0339 | 0.970 | 8.977 | 0.010 | 0.990 | |
| BR | 0.755 | 0.0269 | 0.160 | 0.771 | 0.140 | 0.977 | |
| CS | 1.063 | 0.335 | 0.991 | 1.268 | 0.198 | 0.997 | |
| RH | 1.333 | 1.387 | 0.899 | 1.449 | 0.260 | 0.998 | |
| [1] | 俞盈, 韩兰芳, 姜晓满. 水热炭的制备、结构特征和应用[J]. 环境化学, 2018, 37(6):1232-1244. |
| [2] |
AHMAD M, RAJAPAKSHA A U, LIM J E, et al. Biochar as a sorbent for contaminant management in soil and water: A review[J]. Chemosphere, 2014, 99(3):19-33.
DOI URL |
| [3] |
HAN L, RO K S, SUN K, et al. New evidence for high sorption capacity of hydrochar for hydrophobic organic pollutants[J]. Environmental Science & Technology, 2016, 50(24):13274.
DOI URL |
| [4] | XIE T, REDDY K R, WANG C, et al. Characteristics and applications of biochar for environmental remediation: A review[J]. Critical Reviews in Environmental Science & Technology, 2015, 45(9):939-969. |
| [5] |
LEHMANN J. A handful of carbon[J]. Nature, 2007, 447(7141):143-144.
DOI URL |
| [6] |
ZHU D Q, KWON S, PIGNATELLO J J. Adsorption of single-ring organic compounds to wood charcoals prepared under different thermochemical conditions[J]. Environmental Science & Technology, 2005, 39(11):3990-3998.
DOI URL |
| [7] | 刘雨嫣, 周景尧, 马少强, 等. 水热炭吸附Cr(Ⅵ)热-动力学行为及水热裂解时间的影响[J]. 现代地质, 2017, 31(5):1039-1045. |
| [8] |
LIU Y, MA S, CHEN J. A novel pyro-hydrochar via sequential carbonization of biomass waste: Preparation, characterization and adsorption capacity[J]. Journal of Cleaner Production, 2018, 176:187-195.
DOI URL |
| [9] |
FLORA J F R, LU X, LI L, et al. The effects of alkalinity and acidity of process water and hydrochar washing on the adsorption of atrazine on hydrothermally produced hydrochar[J]. Chemosphere, 2013, 93(9):1989-1996.
DOI URL |
| [10] |
ZHAO X, OUYANG W, HAO F, et al. Properties comparison of biochars from corn straw with different pretreatment and sorption behaviour of atrazine[J]. Bioresource Technology, 2013, 147:338-344.
DOI URL |
| [11] |
HAN L, SUN H, RO K S, et al. Removal of antimony (III) and cadmium (II) from aqueous solution using animal manure-derived hydrochars and pyrochars[J]. Bioresource Technology, 2017, 234:77-85.
DOI URL |
| [12] | STEINER C, TEIXEIRA W G, LEHMANN J, et al. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil[J]. Plant & Soil, 2007, 291(1/2):275-290. |
| [13] |
OLMO M, ALBURQUERQUE J A, BARRON V, et al. Wheat growth and yield responses to biochar addition under Mediterranean climate conditions[J]. Biology and Fertility of Soils, 2014, 50(8):1177-1187.
DOI URL |
| [14] |
ZHANG D, PAN G, WU G, et al. Biochar helps enhance maize productivity and reduce greenhouse gas emissions under balanced fertilization in a rainfed low fertility inceptisol[J]. Chemosphere, 2015, 142:106-113.
DOI URL |
| [15] |
REN J, WANG F, ZHAI Y, et al. Effect of sewage sludge hydrochar on soil properties and Cd immobilization in a contaminated soil[J]. Chemosphere, 2017, 189:627-633.
DOI URL |
| [16] | MARTIN S M, KOOKANA R S, ZWIETEN L V, et al. Marked changes in herbicide sorption-desorption upon ageing of biochars in soil[J]. Journal of Hazardous Materials, 2012, 231:70-78. |
| [17] |
MIAO W, CHENG X Y, MENG J, et al. Ageing of rice husk biochar along a freeze-thaw cycles[J]. MATEC Web of Conferences, 2016, 63:04013.
DOI URL |
| [18] |
WANG H, FENG M, ZHOU F, et al. Effects of atmospheric ageing under different temperatures on surface properties of sludge-derived biochar and metal/metalloid stabilization[J]. Chemosphere, 2017, 184:176-184.
DOI URL |
| [19] | 陈昱, 梁媛, 郑章琪, 等. 老化作用对水稻秸秆生物炭吸附Cd(Ⅱ)能力的影响[J]. 环境化学, 2016, 35(11):2337-2343. |
| [20] |
吴艳姣, 李伟, 吴琼, 等. 水热炭的制备、性质及应用[J]. 化学进展, 2016, 28(1):121-130.
DOI |
| [21] |
LIU Y, SOHI S P, JING F, et al. Oxidative ageing induces change in the functionality of biochar and hydrochar: Mechanistic insights from sorption of atrazine[J]. Environmental Pollution, 2019, 249:1002-1010.
DOI URL |
| [22] |
WIEDNER K, NAISSE C, RUMPEL C, et al. Chemical modification of biomass residues during hydrothermal carbonization-What makes the difference, temperature or feedstock?[J]. Organic Geochemistry, 2013, 54:91-100.
DOI URL |
| [23] |
CHEN G C, SHAN X Q, WANG Y S, et al. Effects of copper, lead, and cadmium on the sorption and desorption of atrazine onto and from carbon nanotubes[J]. Environmental Science & Technology, 2008, 42(22):8297-8302.
DOI URL |
| [24] |
ZHANG L, WANG Q, WANG B, et al. Hydrothermal carbonization of corncob residues for hydrochar production[J]. Energy & Fuels, 2014, 29(2):872-876.
DOI URL |
| [25] |
YANG H, XU R, XUE X, et al. Hybrid surfactant-templated mesoporous silica formed in ethanol and its application for heavy metal removal[J]. Journal of Hazardous Materials, 2008, 152(2):690-698.
DOI URL |
| [26] |
SUN K, TANG J, GONG Y, et al. Characterization of potassium hydroxide (KOH) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water[J]. Environmental Science and Pollution Research, 2015, 22(21):16640-16651.
DOI URL |
| [27] |
CAO X, MA L, GAO B, et al. Dairy-manure derived biochar effectively sorbs lead and atrazine[J]. Environmental Science & Technology, 2009, 43(9):3285-3291.
DOI URL |
| [1] | YANG Meihong, LI Zhixiong, LIU Yuyan, MA Shaoqiang, CHEN Jiawei. Impacts and Mechanisms of Natural Organic Matter and pH on the Transport of Nanobiochar [J]. Geoscience, 2018, 32(01): 113-120. |
| [2] | LIU Yuyan, ZHOU Jingyao, MA Shaoqiang, CHEN Jiawei. Thermodynamic and Kinetic Adsorption of Cr(Ⅵ) on Hydrochars and the Effect of Hydrothermal Time [J]. Geoscience, 2017, 31(05): 1039-1045. |
| [3] | JING Ming, LI Ye, CHEN Ying-yu, CHEN Jia-wei. A Study on Cr(Ⅵ) Migration and Locking in Biochar-amended Soil [J]. Geoscience, 2014, 28(6): 1194-1201. |
| [4] | YUAN Guo-Li, LANG Xin-Xin, SUN Tian-He. A Review of the Research on Persistent Organic Pollutants in Tibetan Plateau [J]. Geoscience, 2012, 26(5): 910-916. |
| [5] | LIU Li-Ya, HE Jiang-Chao, WANG Dun-Jie, ZHANG Cuan. Simulation Study of Hydraulic Connection Between the Polluted River and Groundwater in a Riparian Zone [J]. Geoscience, 2011, 25(6): 1201-1206. |
| [6] | WANG Jun-Jie, HE Jiang-Tao, ZHANG Xin, LI Peng, LIU Yan, LIU Li-Na. Study on Hydrochemistry in a Riparian Site [J]. Geoscience, 2010, 24(5): 1000-1006. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||