



Geoscience ›› 2025, Vol. 39 ›› Issue (02): 248-262.DOI: 10.19657/j.geoscience.1000-8527.2024.133
• Deep-Earth Composition and Metallogenesis • Previous Articles Next Articles
JIN Weiguo1,2( ), YIN Shuo1(
), YIN Shuo1( ), WANG Qingfei1,3, PAN Jiayong1
), WANG Qingfei1,3, PAN Jiayong1
Online:2025-04-10
Published:2025-05-08
Contact:
YIN Shuo
CLC Number:
JIN Weiguo, YIN Shuo, WANG Qingfei, PAN Jiayong. Deciphering Microbial-Mediated Uranium Mineralization via FIB-TEM Nanotomography: A Case Study from the Hailijin Deposit, Songliao Basin[J]. Geoscience, 2025, 39(02): 248-262.
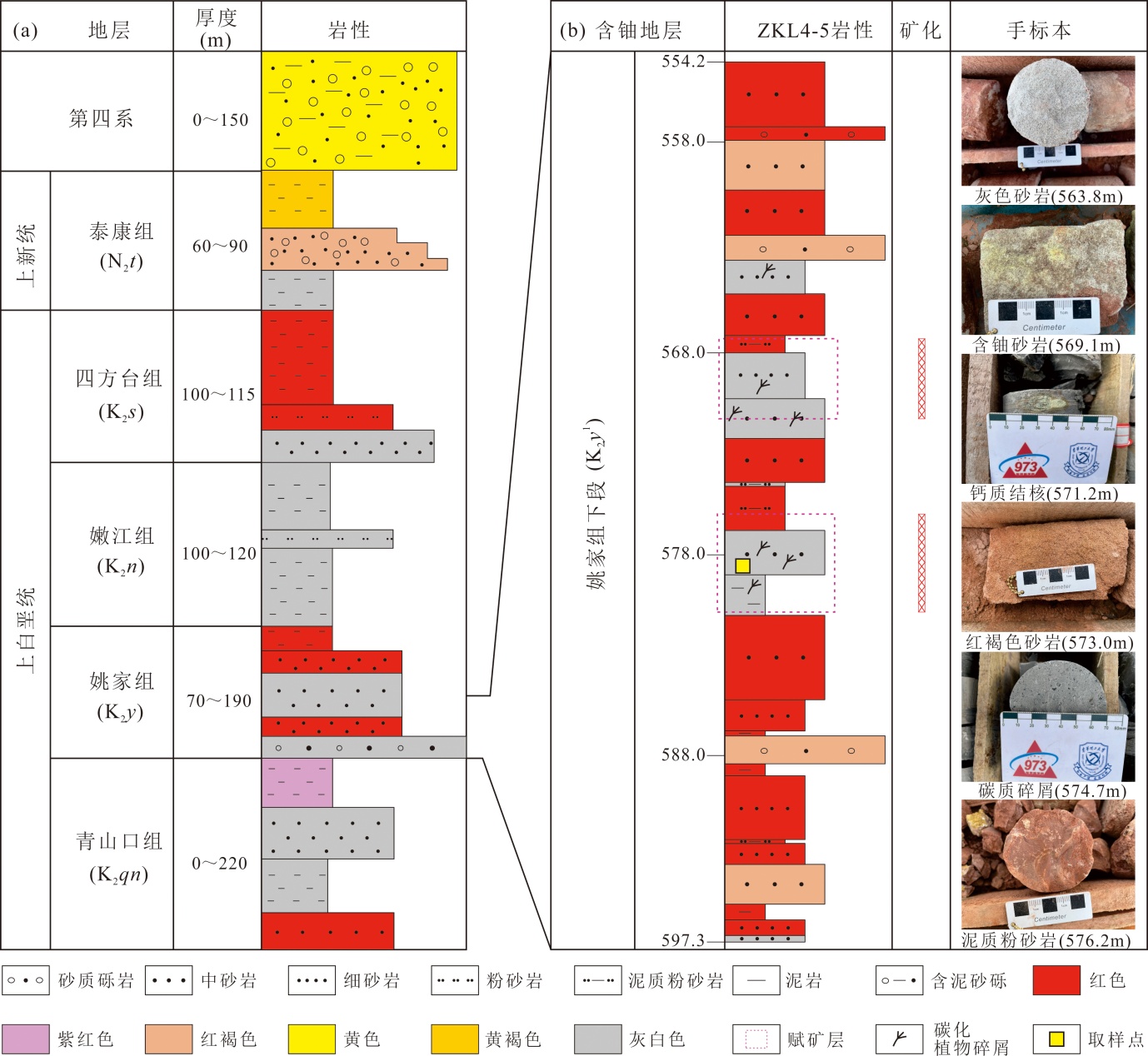
Fig.3 Regional strata, related thickness and ZKL4-5 sedimentary column of drill core in the Hailijin uranium deposit (modified from references [12-13])
| 主量元素 (%) | 样品编号 | 最大值 | 最小值 | 平均值 | ||||
|---|---|---|---|---|---|---|---|---|
| 1-1 | 1-2 | 1-3 | 1-4 | 1-5 | ||||
| SiO2 | 0.27 | 0.26 | 0.31 | - | - | 0.31 | 0.26 | 0.27 |
| ThO2 | 3.87 | 3.18 | 3.43 | 4.18 | 3.72 | 4.18 | 3.`18 | 3.72 |
| UO2 | 0.32 | 0.30 | 0.36 | 0.26 | 0.25 | 0.36 | 0.25 | 0.30 |
| P2O5 | 29.00 | 29.05 | 29.38 | 28.84 | 29.00 | 29.38 | 28.84 | 29.00 |
| La2O3 | 11.45 | 11.20 | 10.73 | 10.34 | 10.70 | 11.45 | 10.34 | 10.73 |
| Ce2O3 | 33.56 | 32.53 | 34.30 | 38.64 | 37.82 | 38.64 | 32.53 | 34.30 |
| Pr2O3 | 4.06 | 3.20 | 3.87 | 3.36 | 3.75 | 4.06 | 3.20 | 3.75 |
| Nd2O3 | 11.87 | 11.50 | 12.95 | 11.53 | 11.22 | 12.95 | 11.22 | 11.53 |
| Sm2O3 | 2.36 | 2.06 | 2.60 | 1.58 | 1.73 | 2.60 | 1.58 | 2.06 |
| Eu2O3 | 0.62 | 0.81 | 0.54 | 0.69 | 0.76 | 0.81 | 0.54 | 0.69 |
| Gd2O3 | 1.29 | 2.30 | 1.36 | 0.73 | 0.70 | 2.30 | 0.70 | 1.29 |
| Dy2O3 | 0.78 | 0.82 | 0.09 | 0.05 | 0.23 | 0.82 | 0.05 | 0.23 |
| Y2O3 | 1.11 | 1.32 | 0.21 | 0.16 | 0.12 | 1.32 | 0.12 | 0.21 |
| Lu2O3 | - | 0.05 | 0.14 | 0.19 | 0.20 | 0.20 | 0.05 | 0.17 |
| SO3 | 0.03 | 0.03 | - | 0.01 | 0.02 | 0.03 | 0.01 | 0.03 |
| CaO | 0.80 | 0.81 | 0.80 | 0.04 | - | 0.81 | 0.04 | 0.80 |
| Total | 101.39 | 99.44 | 101.50 | 100.59 | 100.20 | 101.50 | 99.44 | 100.59 |
| Th/U | 12.01 | 10.50 | 9.60 | 16.14 | 15.11 | 16.14 | 9.60 | 12.01 |
| 主量元素 (%) | 样品编号 | 最大值 | 最小值 | 平均值 | ||||
| 2-1 | 2-2 | 2-3 | 2-4 | 2-5 | ||||
| SiO2 | 0.28 | 0.26 | 0.24 | 0.29 | 0.19 | 0.29 | 0.19 | 0.26 |
| ThO2 | 4.00 | 4.08 | 4.03 | 4.21 | 4.09 | 4.21 | 4.00 | 4.08 |
| UO2 | 0.06 | 0.05 | 0.10 | 0.05 | 0.10 | 0.10 | 0.05 | 0.06 |
| P2O5 | 28.85 | 28.84 | 28.75 | 28.51 | 28.67 | 28.85 | 28.51 | 28.75 |
| La2O3 | 11.15 | 10.82 | 11.09 | 11.22 | 12.82 | 12.82 | 10.82 | 11.15 |
| Ce2O3 | 31.15 | 32.10 | 31.35 | 32.24 | 33.79 | 33.79 | 31.15 | 32.10 |
| Pr2O3 | 3.97 | 4.05 | 3.87 | 3.60 | 4.03 | 4.05 | 3.60 | 3.97 |
| Nd2O3 | 12.51 | 12.52 | 11.61 | 12.26 | 11.29 | 12.52 | 11.29 | 12.26 |
| Sm2O3 | 2.32 | 2.11 | 2.43 | 2.25 | 1.88 | 2.43 | 1.88 | 2.25 |
| Eu2O3 | 0.66 | 0.44 | 0.80 | 0.61 | 0.62 | 0.80 | 0.44 | 0.62 |
| Gd2O3 | 1.75 | 1.62 | 2.14 | 1.50 | 1.95 | 2.14 | 1.50 | 1.75 |
| Dy2O3 | 0.35 | 0.42 | - | 0.67 | 0.18 | 0.67 | 0.18 | 0.39 |
| Y2O3 | 1.67 | 1.85 | 2.06 | 1.69 | 1.31 | 2.06 | 1.31 | 1.69 |
| Lu2O3 | 0.20 | 0.10 | 0.10 | - | - | 0.20 | 0.10 | 0.10 |
| SO3 | - | - | - | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| CaO | 0.80 | 0.77 | 0.91 | 0.79 | 0.51 | 0.91 | 0.51 | 0.79 |
| Total | 99.73 | 100.03 | 99.47 | 99.90 | 100.45 | 100.45 | 99.47 | 99.90 |
| Th/U | 64.52 | 75.46 | 41.52 | 84.26 | 42.65 | 84.26 | 41.52 | 64.52 |
Table 1 Major and trace element concentrations of monazite by electron probe microanalysis (EPMA)
| 主量元素 (%) | 样品编号 | 最大值 | 最小值 | 平均值 | ||||
|---|---|---|---|---|---|---|---|---|
| 1-1 | 1-2 | 1-3 | 1-4 | 1-5 | ||||
| SiO2 | 0.27 | 0.26 | 0.31 | - | - | 0.31 | 0.26 | 0.27 |
| ThO2 | 3.87 | 3.18 | 3.43 | 4.18 | 3.72 | 4.18 | 3.`18 | 3.72 |
| UO2 | 0.32 | 0.30 | 0.36 | 0.26 | 0.25 | 0.36 | 0.25 | 0.30 |
| P2O5 | 29.00 | 29.05 | 29.38 | 28.84 | 29.00 | 29.38 | 28.84 | 29.00 |
| La2O3 | 11.45 | 11.20 | 10.73 | 10.34 | 10.70 | 11.45 | 10.34 | 10.73 |
| Ce2O3 | 33.56 | 32.53 | 34.30 | 38.64 | 37.82 | 38.64 | 32.53 | 34.30 |
| Pr2O3 | 4.06 | 3.20 | 3.87 | 3.36 | 3.75 | 4.06 | 3.20 | 3.75 |
| Nd2O3 | 11.87 | 11.50 | 12.95 | 11.53 | 11.22 | 12.95 | 11.22 | 11.53 |
| Sm2O3 | 2.36 | 2.06 | 2.60 | 1.58 | 1.73 | 2.60 | 1.58 | 2.06 |
| Eu2O3 | 0.62 | 0.81 | 0.54 | 0.69 | 0.76 | 0.81 | 0.54 | 0.69 |
| Gd2O3 | 1.29 | 2.30 | 1.36 | 0.73 | 0.70 | 2.30 | 0.70 | 1.29 |
| Dy2O3 | 0.78 | 0.82 | 0.09 | 0.05 | 0.23 | 0.82 | 0.05 | 0.23 |
| Y2O3 | 1.11 | 1.32 | 0.21 | 0.16 | 0.12 | 1.32 | 0.12 | 0.21 |
| Lu2O3 | - | 0.05 | 0.14 | 0.19 | 0.20 | 0.20 | 0.05 | 0.17 |
| SO3 | 0.03 | 0.03 | - | 0.01 | 0.02 | 0.03 | 0.01 | 0.03 |
| CaO | 0.80 | 0.81 | 0.80 | 0.04 | - | 0.81 | 0.04 | 0.80 |
| Total | 101.39 | 99.44 | 101.50 | 100.59 | 100.20 | 101.50 | 99.44 | 100.59 |
| Th/U | 12.01 | 10.50 | 9.60 | 16.14 | 15.11 | 16.14 | 9.60 | 12.01 |
| 主量元素 (%) | 样品编号 | 最大值 | 最小值 | 平均值 | ||||
| 2-1 | 2-2 | 2-3 | 2-4 | 2-5 | ||||
| SiO2 | 0.28 | 0.26 | 0.24 | 0.29 | 0.19 | 0.29 | 0.19 | 0.26 |
| ThO2 | 4.00 | 4.08 | 4.03 | 4.21 | 4.09 | 4.21 | 4.00 | 4.08 |
| UO2 | 0.06 | 0.05 | 0.10 | 0.05 | 0.10 | 0.10 | 0.05 | 0.06 |
| P2O5 | 28.85 | 28.84 | 28.75 | 28.51 | 28.67 | 28.85 | 28.51 | 28.75 |
| La2O3 | 11.15 | 10.82 | 11.09 | 11.22 | 12.82 | 12.82 | 10.82 | 11.15 |
| Ce2O3 | 31.15 | 32.10 | 31.35 | 32.24 | 33.79 | 33.79 | 31.15 | 32.10 |
| Pr2O3 | 3.97 | 4.05 | 3.87 | 3.60 | 4.03 | 4.05 | 3.60 | 3.97 |
| Nd2O3 | 12.51 | 12.52 | 11.61 | 12.26 | 11.29 | 12.52 | 11.29 | 12.26 |
| Sm2O3 | 2.32 | 2.11 | 2.43 | 2.25 | 1.88 | 2.43 | 1.88 | 2.25 |
| Eu2O3 | 0.66 | 0.44 | 0.80 | 0.61 | 0.62 | 0.80 | 0.44 | 0.62 |
| Gd2O3 | 1.75 | 1.62 | 2.14 | 1.50 | 1.95 | 2.14 | 1.50 | 1.75 |
| Dy2O3 | 0.35 | 0.42 | - | 0.67 | 0.18 | 0.67 | 0.18 | 0.39 |
| Y2O3 | 1.67 | 1.85 | 2.06 | 1.69 | 1.31 | 2.06 | 1.31 | 1.69 |
| Lu2O3 | 0.20 | 0.10 | 0.10 | - | - | 0.20 | 0.10 | 0.10 |
| SO3 | - | - | - | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| CaO | 0.80 | 0.77 | 0.91 | 0.79 | 0.51 | 0.91 | 0.51 | 0.79 |
| Total | 99.73 | 100.03 | 99.47 | 99.90 | 100.45 | 100.45 | 99.47 | 99.90 |
| Th/U | 64.52 | 75.46 | 41.52 | 84.26 | 42.65 | 84.26 | 41.52 | 64.52 |
| [1] | TIEH T T, LEDGER E B, ROWE M W. Release of uranium from granitic rocks during in situ weathering and initial erosion (central Texas)[J]. Chemical Geology, 1980, 29(1/2/3/4): 227-248. |
| [2] | ZIELINSKI R A. Uranium mobility during interaction of rhyolitic obsidian, perlite and felsite with alkaline carbonate solution: T=120 ℃, P=210 kg/cm2[J]. Chemical Geology, 1979, 27(1/2): 47-63. |
| [3] | ZIELINSKI R A. Tuffaceous sediments as source rocks for uranium: A case study of the White River Formation, Wyoming[J]. Journal of Geochemical Exploration, 1983, 18(3): 285-306. |
| [4] | ROSHOLT J N, NOBLE D C. Loss of uranium from crystallized silicic volcanic rocks[J]. Earth and Planetary Science Letters, 1969, 6(4): 268-270. |
| [5] | MOYEN J F, CUNEY M, BARATOUX D, et al. Multi-scale spatial distribution of K, Th and U in an Archaean potassic granite: A case study from the Heerenveen batholith, Barberton Granite-Greenstone Terrain, South Africa[J]. South African Journal of Geology, 2021, 124(1): 53-86. |
| [6] | GUTHRIE V A, KLEEMAN J D. Changing uranium distributions during weathering of granite[J]. Chemical Geology, 1986, 54(1/2): 113-126. |
| [7] | 臧亚辉, 李继木, 宁君, 等. 松辽盆地南部海力锦地区上白垩统姚家组砂岩物源示踪及其构造背景综合研究:来自岩石地球化学及锆石U-Pb年代学的制约[J]. 地质科技通报, 2023, 42(5): 175-190. |
| [8] | 田明明, 李子颖, 张云龙, 等. 松辽盆地海力锦铀矿床黄铁矿微量元素、硫同位素特征及其对成矿流体性质的指示[J]. 铀矿地质, 2024, 40(1): 119-128. |
| [9] | 宁君, 李继木, 夏菲, 等. 松辽盆地南部海力锦铀矿床地质特征及成因探讨[J]. 东华理工大学学报(自然科学版), 2023, 46(3): 223-232. |
| [10] | 宁君, 李继木, 卢天军, 等. 松辽盆地南部海力锦铀矿床姚家组下段黏土矿物特征及其铀成矿意义[J]. 铀矿地质, 2023, 39(2): 188-201. |
| [11] | 臧亚辉, 姜山, 李继木, 等. 松辽盆地南部海力锦铀矿床铀矿物赋存状态及富集机理:试论“潮汐式”成矿作用过程[J]. 大地构造与成矿学, 2023, 47(3): 546-569. |
| [12] | TIAN M M, LI Z Y, ZHANG Y L, et al. Genetic mechanism of tabular-shaped orebody of the hailijin sandstone-type uranium deposit in the Songliao basin: Constraints on the clay mineralogy of ore-bearing sandstone[J]. Minerals, 2023, 13(10): 1324. |
| [13] | YAN Z B, ZHANG W W, XIA F, et al. Uranium pre-concentration in sandstone-hosted U deposits: A case study from the hailijin ore field, SW Songliao basin, NE China[J]. Ore Geology Reviews, 2023, 161: 105661. |
| [14] | YIN S, YAN Z B, FU J L, et al. Polymorphic transformations of titanium oxides contribute to economic uranium mineralization in sandstone[J]. Geology, 2024, 52(7): 481-485. |
| [15] | 张红雨, 杨立明, 苏犁, 等. LA-ICP-MS独居石的U(Th)-Pb年龄精确测定方法及地质意义探究[J]. 现代地质, 2023, 37(2): 443-462. |
| [16] | 李厚民, 李立兴, 李以科, 等. 内蒙古白云鄂博铁-铌-稀土矿床矿化蚀变矿物组合及流体组成[J]. 现代地质, 2024, 38(1): 13-24. |
| [17] | 宓奎峰, 杨艳, 颜廷杰, 等. 内蒙古沙麦钨矿区高分异花岗岩独居石U-Pb定年及成矿意义[J]. 现代地质, 2020, 34(3): 504-513. |
| [18] | HECHT L, CUNEY M. Hydrothermal alteration of monazite in the Precambrian crystalline basement of the Athabasca Basin (Saskatchewan, Canada): Implications for the formation of unconformity-related uranium deposits[J]. Mineralium Deposita, 2000, 35(8): 791-795. |
| [19] | WAGANI I, PAGEL M, JANOTS E, et al. Detrital monazite in the tim mersoi basin, Niger: Provenance and contribution to the uranium budget in siliciclastic sediments[J]. The Canadian Mineralogist, 2011, 49(2): 487-501. |
| [20] | AMBOH TIFANG J. Preliminary survey for uranium in Cretaceous sandstones of bonako-sole areas, ngondo complex, southwestern Cameroon[J]. Earth Sciences, 2017, 6(1): 1. |
| [21] | 王海涛. 开鲁坳陷姚家组下段沉积特征与铀成矿作用[J]. 东华理工大学学报(自然科学版), 2022, 45(3): 223-233. |
| [22] | 吴根耀. 白垩纪:中国及邻区板块构造演化的一个重要变换期[J]. 中国地质, 2006, 33(1): 64-77. |
| [23] | REN J Y, TAMAKI K, LI S T, et al. Late Mesozoic and Cenozoic rifting and its dynamic setting in Eastern China and adjacent areas[J]. Tectonophysics, 2002, 344(3/4): 175-205. |
| [24] | LI Z Q, CHEN J L, ZOU H, et al. Mesozoic-Cenozoic tectonic evolution and dynamics of the Songliao Basin, NE Asia: Implications for the closure of the Paleo-Asian Ocean and Mongol-Okhotsk Ocean and subduction of the Paleo-Pacific Ocean[J]. Earth-Science Reviews, 2021, 218: 103471-108252. |
| [25] | WANG P J, MATTERN F, DIDENKO N A, et al. Tectonics and cycle system of the Cretaceous Songliao basin: An inverted active continental margin basin[J]. Earth-Science Reviews, 2016, 159(1): 82-102. |
| [26] | SONG Y, STEPASHKO A, LIU K Y, et al. Post-rift tectonic history of the Songliao basin, NE China: Cooling events and post-rift unconformities driven by orogenic pulses from plate boundaries[J]. Journal of Geophysical Research, 2018, 123(3): 2363-2395. |
| [27] | 宁君, 夏菲, 聂逢君, 等. 浅析松辽盆地南部姚下段灰色砂体与铀成矿关系[J]. 东华理工大学学报(自然科学版), 2018, 41(4): 336-342. |
| [28] | KETTANAH Y A, ISMAIL S A. Heavy mineral concentrations in the sandstones of Amij Formation with particular emphasis on the mineral chemistry and petrographic characteristics of monazite, western desert of Iraq[J]. Journal of African Earth Sciences, 2016, 123: 350-369. |
| [29] | LINTHOUT K. Tripartite division of the system 2REPPO4-CaTh(PO4)2-2ThSiO4, discreditation of brabantite, and recognition of cheralite as the Name for members dominated by CaTh(PO4)2[J]. The Canadian Mineralogist, 2007, 45(3): 503-508. |
| [30] | MORTON A C, HALLSWORTH C. Chapter 7 stability of detrital heavy minerals during burial diagenesis[M]// Heavy Minerals in Use. Amsterdam: Elsevier, 2007: 215-245. |
| [31] | ALLAZ J, SELLECK B, WILLIAMS M L, et al. Microprobe analysis and dating of monazite from the Potsdam formation, New York: A progressive record of chemical reaction and fluid interaction[J]. American Mineralogist, 2013, 98(7): 1106-1119. |
| [32] | RICHARD A, MONTEL J M, LEBORGNE R, et al. Monazite alteration in H2O±HCl±NaCl±CaCl2 fluids at 150 ℃ and psat: Implications for uranium deposits[J]. Minerals, 2015, 5(4): 693-706. |
| [33] | ZHAO L, CAI C F, JIN R S, et al. Mineralogical and geochemical evidence for biogenic and petroleum-related uranium mineralization in the Qianjiadian deposit, NE China[J]. Ore Geology Reviews, 2018, 101: 273-292. |
| [34] | BHATTACHARYYA A, CAMPBELL K M, KELLY S D, et al. Biogenic non-crystalline U(IV) revealed as major component in uranium ore deposits[J]. Nature Communications, 2017, 8(1): 15538. |
| [35] | WORTMANN U G, BERNASCONI S M, BÖTTCHER M E. Hypersulfidic deep biosphere indicates extreme sulfur isotope fractionation during single-step microbial sulfate reduction[J]. Geology, 2001, 29(7): 647. |
| [36] |
SHEN J B, YUAN L X, ZHANG J L, et al. Phosphorus dynamics: From soil to plant[J]. Plant Physiology, 2011, 156(3): 997-1005.
DOI PMID |
| [37] |
CORBETT M K, EKSTEEN J J, NIU X Z, et al. Interactions of phosphate solubilising microorganisms with natural rare-earth phosphate minerals: A study utilizing Western Australian monazite[J]. Bioprocess and Biosystems Engineering, 2017, 40(6): 929-942.
DOI PMID |
| [38] |
BRISSON V L, ZHUANG W Q, ALVAREZ COHEN L.Bioleaching of rare earth elements from monazite sand[J]. Biotechnology and Bioengineering, 2016, 113(2): 339-348.
DOI PMID |
| [39] | HE Y L, MA L Y, LIANG X L, et al. Resistant rare earth phosphates as possible sources of environmental dissolved rare earth elements: Insights from experimental bio-weathering of xenotime and monazite[J]. Chemical Geology, 2024, 661(9): 122186. |
| [40] | SON H J, PARK G T, CHA M S, et al. Solubilization of insoluble inorganic phosphates by a novel salt- and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere[J]. Bioresource Technology, 2006, 97(2): 204-210. |
| [41] | NI Y X, HUGHES J M, MARIANO A N. Crystal chemistry of the monazite and xenotime structures[J]. American Mineralogist, 1995, 80(1/2): 21-26. |
| [42] | FOUGEROUSE D, REDDY S M, SEYDOUX-GUILLAUME A M, et al. Mechanical twinning of monazite expels radiogenic lead[J]. Geology, 2021, 49(4): 417-421. |
| [43] | SHEN Y H, ZHENG X Y, WANG X Y, et al. The biomineralization process of uranium(VI) by Saccharomyces cerevisiae-transformation from amorphous U(VI) to crystalline chernikovite[J]. Applied Microbiology and Biotechnology, 2018, 102(9): 4217-4229. |
| [44] | ZHENG X Y, SHEN Y H, WANG X Y, et al. Effect of pH on uranium(VI) biosorption and biomineralization by Saccharomyces cerevisiae[J]. Chemosphere, 2018, 203: 109-116. |
| [45] | WANG P P, DONG F Q, WANG X H, et al. Effects of riboflavin and AQS as electron shuttles on U(vi) reduction and precipitation by Shewanella putrefaciens[J]. RSC Advances, 2018, 8(54): 30692-30700. |
| [46] | HU N, LI K, SUI Y, et al. Utilization of phosphate rock as a sole source of phosphorus for uranium biomineralization mediated by Penicillium funiculosum[J]. RSC Advances, 2018, 8(24): 13459-13465. |
| [47] | YU Q H, YUAN Y H, FENG L J, et al. Highly efficient immobilization of environmental uranium contamination with Pseudomonas stutzeri by biosorption, biomineralization, and bioreduction[J]. Journal of Hazardous Materials, 2022, 424: 127758. |
| [48] | ZHENG X Y, WANG X Y, SHEN Y H, et al. Biosorption and biomineralization of uranium(VI) by saccharomyces cerevisiae:Crystal formation of chernikovite[J]. Chemosphere, 2017, 175: 161-169. |
| [49] | NIE X, DONG F, BIAN L, et al. Uranium Binding on Landoltia punctata as a Result of Formation of Insoluble Nano-U (VI) and U (IV) Phosphate Minerals[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(2): 1494-1502. |
| [50] | 沈扬皓. 啤酒酵母和拟棘壳孢属菌与铀的相互作用机理研究[D]. 兰州: 兰州大学, 2021. |
| [51] | TANG X, LI Q L, ZHANG B, et al. The chemical state and occupancy of radiogenic Pb, and crystallinity of RW-1 monazite revealed by XPS and TEM[J]. Minerals, 2020, 10(6): 504. |
| [52] | SHIN D, KIM J, KIM B S, et al. Use of phosphate solubilizing bacteria to leach rare earth elements from monazite-bearing ore[J]. Minerals, 2015, 5(2): 189-202. |
| [53] | XIE J C, LIN J F, ZHOU X H. pH-dependent microbial reduction of uranium(VI) in carbonate-free solutions: UV-vis, XPS, TEM, and thermodynamic studies[J]. Environmental Science and Pollution Research, 2018, 25(22): 22308-22317. |
| [54] | FANIZZA M F, YOON H, ZHANG C Y, et al. Pore-scale evaluation of uranyl phosphate precipitation in a model groundwater system[J]. Water Resources Research, 2013, 49(2): 874-890. |
| [55] | SINGH A, ULRICH K U, GIAMMAR D E. Impact of phosphate on U(VI) immobilization in the presence of goethite[J]. Geochimica et Cosmochimica Acta, 2010, 74(22): 6324-6343. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||